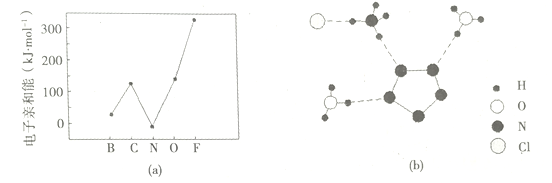
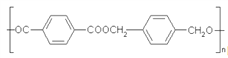
��Ŀ����
����Ŀ���������¼����л���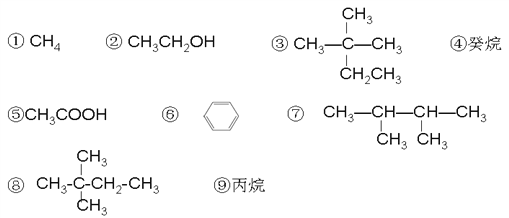
�������������������ʰ�Ҫ��ش��������⣺
��1����Է�������Ϊ44�������ṹ��ʽΪ __________��
��2�������к���14����ԭ�ӵ������ķ���ʽ��________��
��3����ۻ�Ϊͬ���칹����� __________������ţ���
��4������������ζ��������ȡ�����л�����������������������Һ�巢��һ�����Ӧ�Ļ�ѧ����ʽ _____________________ ��
��5���ߵ�ͬ���칹�壨�����ǿռ��칹����һ�ȴ�����3��,����дһ��__________ ��
��6���л�����ڼ��������º�O2��Ӧ�Ļ�ѧ����ʽ _______________ ��
��7����120����1.01��105Pa�����£�ij����̬����������O2��ȫ��Ӧ��÷�Ӧǰ����������û�з����ı䣬�������____������ţ�������Ϊ ________ ��ϵ��
��8���л���ݺ͢���һ�������·�����Ӧ�Ļ�ѧ����ʽ��________________ ��
���𰸡� CH3CH2CH3 C6H14 �� ![]() CH3CH2C(CH3)3 2CH3CH2OH��O2
CH3CH2C(CH3)3 2CH3CH2OH��O2 ![]() 2CH3CHO��2H2O �� ͬϵ��
2CH3CHO��2H2O �� ͬϵ�� ![]()
����������1��.�����ķ���ͨʽΪ��CnH(2n+2)����14n+2=44�����n=3��������Է�������Ϊ44�������DZ��飬��ṹ��ʽΪCH3CH2CH3���ʴ�Ϊ��CH3CH2CH3��
��2��.�����ķ���ͨʽΪ��CnH(2n+2)����2n+2=14�����n=6�����Է����к���14����ԭ�ӵ������Ǽ��飬�����ʽΪC6H14���ʴ�Ϊ��C6H14��
��3��. ���ķ���ʽΪC6H14���ߵķ���ʽҲ��C6H14�����߷���ʽ��ͬ���ṹ��ͬ����Ϊͬ���칹�壬�ʴ�Ϊ���ߣ�
��4��.����Ŀ�����������У�����������ζ��������ȡ�����л����DZ�������������������������Һ�巢��һ�����Ӧ�����屽���廯�⣬��ѧ����ʽΪ��![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
��5��.�ߵ�ͬ���칹�壨�����ǿռ��칹����һ�ȴ�����3�֣�˵����ͬ���칹���к������ֵ�Ч��ԭ�ӣ�������CH3CH2C(CH3)3���ʴ�Ϊ��CH3CH2C(CH3)3��
��6��.�Ҵ��ڼ�����������������Ӧ������ȩ��ˮ����ѧ����ʽΪ��2CH3CH2OH��O2 ![]() 2CH3CHO��2H2O���ʴ�Ϊ��2CH3CH2OH��O2
2CH3CHO��2H2O���ʴ�Ϊ��2CH3CH2OH��O2 ![]() 2CH3CHO��2H2O��
2CH3CHO��2H2O��
��7��.��120����1.01��105Pa�����£����ɵ�ˮΪ��̬����CxHy+��x+![]() ��O2
��O2![]() xCO2+
xCO2+![]() H2O��g������1+��x+
H2O��g������1+��x+![]() ��=x+
��=x+![]() �����y=4��������ʽ����ԭ����ĿΪ4��Ϊ���飬����Ϊͬϵ��ʴ�Ϊ���٣�ͬϵ�
�����y=4��������ʽ����ԭ����ĿΪ4��Ϊ���飬����Ϊͬϵ��ʴ�Ϊ���٣�ͬϵ�
��8��.�������Ҵ���һ�������·���������Ӧ��������������ˮ����Ӧ����ʽΪ��![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��

 �����Ļ�������ҵϵ�д�
�����Ļ�������ҵϵ�д�