��Ŀ����
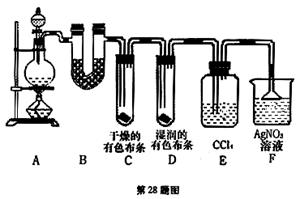
(14��) ��ͼ��ʾ��һ����ȡCl2����Cl2Ϊԭ�Ͻ����ض���Ӧ��װ�ã����и��Լ�ƿ��װ�Լ�Ϊ��B(Na2S)��C(FeBr2)��D(����-KI)��E(SO2��BaCl2)��F(ˮ)��H(��ɫʯ��)
(1)д��ʵ������ȡCl2�Ļ�ѧ����ʽ___________________________________________________________
(2)����bƿ�ڼ����Һ���� ��װ�â�����ͼ�е�װ��_______(����)����
(3)ʵ�鿪ʼʱ���ȵ�ȼA���ƾ��ƣ���Һ©�������͢�������Cl2��������װ�ã��ٵ�ȼG���ƾ��ƣ��ش��������⣺
������װ���е�������B _________________��D _______________________ __
��д��E�з�Ӧ�����ӷ���ʽ��
_____________________________________��________________________________________
(4)G��Ӳ�ʲ�������ʢ��̼�ۣ���Ӧ��IJ���ΪCO2��HCl��д��G�еķ�Ӧ�Ļ�ѧ����ʽ__________
(5)��H������ɫʯ����Һ����ɫ����ɫ��Ϊ��ɫ���ٱ�Ϊ��ɫ����ԭ����__________________________

(1)д��ʵ������ȡCl2�Ļ�ѧ����ʽ___________________________________________________________
(2)����bƿ�ڼ����Һ���� ��װ�â�����ͼ�е�װ��_______(����)����
(3)ʵ�鿪ʼʱ���ȵ�ȼA���ƾ��ƣ���Һ©�������͢�������Cl2��������װ�ã��ٵ�ȼG���ƾ��ƣ��ش��������⣺
������װ���е�������B _________________��D _______________________ __
��д��E�з�Ӧ�����ӷ���ʽ��
_____________________________________��________________________________________
(4)G��Ӳ�ʲ�������ʢ��̼�ۣ���Ӧ��IJ���ΪCO2��HCl��д��G�еķ�Ӧ�Ļ�ѧ����ʽ__________
(5)��H������ɫʯ����Һ����ɫ����ɫ��Ϊ��ɫ���ٱ�Ϊ��ɫ����ԭ����__________________________
(14��) ��1��MnO2��4HCl(Ũ) MnCl2��H2O��Cl2�� ��2������ʳ��ˮ ��
MnCl2��H2O��Cl2�� ��2������ʳ��ˮ ��
��3������dz��ɫ�������ɣ���Һ����
��Cl2��SO2��2H2O=4H+��SO42-��2Cl- Ba2+��SO42-=BaSO4��
��4�� C��2H2O��2Cl2 CO2����4HCl��
CO2����4HCl��
��5��Cl2��H2O��Ӧ���ɵ�HClʹ��ɫʯ����Һ���ɫ��ʣ���Cl2����ˮ��������HClO������Ư�����ã�ʹ��ɫ��ȥ
 MnCl2��H2O��Cl2�� ��2������ʳ��ˮ ��
MnCl2��H2O��Cl2�� ��2������ʳ��ˮ ����3������dz��ɫ�������ɣ���Һ����
��Cl2��SO2��2H2O=4H+��SO42-��2Cl- Ba2+��SO42-=BaSO4��
��4�� C��2H2O��2Cl2
 CO2����4HCl��
CO2����4HCl����5��Cl2��H2O��Ӧ���ɵ�HClʹ��ɫʯ����Һ���ɫ��ʣ���Cl2����ˮ��������HClO������Ư�����ã�ʹ��ɫ��ȥ
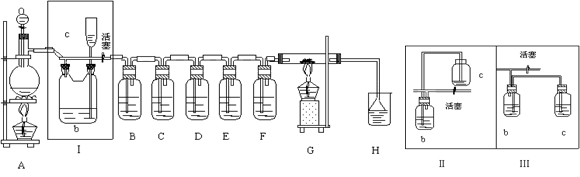
�����������1��ʵ������ȡ�����ķ���ʽ��MnO2��4HCl(Ũ)
 MnCl2��H2O��Cl2����
MnCl2��H2O��Cl2������2������Ũ������лӷ��ԣ��������ɵ������к����Ȼ������壬����bƿ�ڼ����Һ���DZ���ʳ��ˮ��Ŀ���dz�ȥ�����е��Ȼ������塣װ�â����������ȫƿ�����ã����Ի�������ͼ�е�װ��Ӧ���Ǣ�
��3������������ǿ�����ԣ��������������ɵ���S������B����dz��ɫ�������ɣ������ܰѵ⻯���������ɵ��ʵ⣬��������������ɫ��
�����������ܰ�SO2�����������ᣬ�����������ᱵ��ɫ����������E�з�Ӧ�����ӷ���ʽ��Cl2��SO2��2H2O=4H+��SO42-��2Cl-��Ba2+��SO42-=BaSO4����
��4�������������֪����ˮ�μӣ����Է�Ӧ�ķ���ʽ��C��2H2O��2Cl2
 CO2����4HCl����
CO2����4HCl������5������Cl2��H2O��Ӧ���ɵ�HClʹ��ɫʯ����Һ���ɫ��ʣ���Cl2����ˮ��������HClO������Ư�����ã�����ʹ��ɫ��ȥ��
��������ѧ��һ����ʵ��Ϊ������ѧ�ƣ������л�ѧʵ�鼴��ѧ̽��֮˵�����ݹ۽�����߿�����Ҫ�Կ���̽����ʵ��������Ʊ�ʵ��Ϊ������Щ̽���Ժ��Ʊ���ʵ������⣬�ۺ���ǿ�����ۺ�ʵ������ϵ���ܣ��еĻ��ṩһЩ�µ���Ϣ��Ҫ���������侲�����⣬��ϵ��ѧ����֪ʶ�ͼ��ܣ�����֪ʶ����ȡ�Ǩ�ơ����飬ȫ��ϸ�µ�˼��������ȷ����

��ϰ��ϵ�д�
�����Ŀ