��Ŀ����
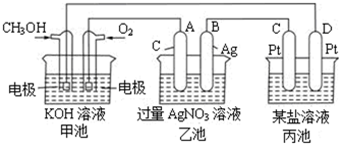
��ͼ��һ���绯ѧ���̵�ʾ��ͼ��
��ش��������⣺
��1��ͼ�м׳��� ���ԭ��ء������ء���Ƴء�����
��2��A��ʯī���缫�������� �������������������������������������
��3��д��ͨ��CH3OH�ĵ缫�ĵ缫��Ӧʽ
��4���ҳ��з�Ӧ�Ļ�ѧ����ʽΪ ���ҳ���B��Ag������������5.4g���׳�������������O2�����Ϊ L����״��������ʱ������ij�缫����1.6gij������������е�ij����Һ������
A��MgSO4B��CuSO4 C��NaClD��AgNO3��

��ش��������⣺
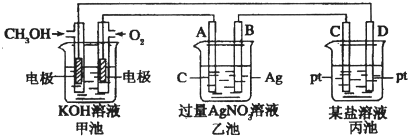
��1��ͼ�м׳���
��2��A��ʯī���缫��������
��3��д��ͨ��CH3OH�ĵ缫�ĵ缫��Ӧʽ
��4���ҳ��з�Ӧ�Ļ�ѧ����ʽΪ
A��MgSO4B��CuSO4 C��NaClD��AgNO3��
��������1���׳����Է��Ľ���������ԭ��Ӧ����������ԭ��أ�
��2�����ǵ��غ�ͨ��������������Ϊ������
��3��ȼ�ϵ���У�������ͨ��ȼ�ϣ�ȼ��ʧ���ӷ���������Ӧ��
��4���ҳ��ǵ��أ�̼���������������������Է�Ӧ�ǵ����������Һ������ת�Ƶ����غ������������ ������������У���������������������ת�Ƶ��Ӽ�����������ԭ���������Ӷ�ȷ���Σ�
��2�����ǵ��غ�ͨ��������������Ϊ������
��3��ȼ�ϵ���У�������ͨ��ȼ�ϣ�ȼ��ʧ���ӷ���������Ӧ��
��4���ҳ��ǵ��أ�̼���������������������Է�Ӧ�ǵ����������Һ������ת�Ƶ����غ������������ ������������У���������������������ת�Ƶ��Ӽ�����������ԭ���������Ӷ�ȷ���Σ�
����⣺��1���׳����Է��Ľ���������ԭ��Ӧ����������ԭ��أ��ʴ�Ϊ��ԭ��أ�
��2�����ǵ��أ���Ϊԭ��أ�ͨ��������������A������������Ϊ�����е��������ʴ�Ϊ��������
��3��ȼ�ϵ���У�������ͨ��ȼ�ϣ����������£��״�ˮ�к����������ӷ�Ӧ����̼������Ӻ�ˮ�����Ե缫��ӦʽΪ��CH3OH+8OH--6e-=CO32-+6H2O��
�ʴ�Ϊ��CH3OH+8OH--6e-=CO32-+6H2O��
��4���ҳ��ǵ��أ�̼���������������������Է�Ӧ�ǵ����������Һ����ط�ӦʽΪ��4AgNO3+2H2O
4Ag+O2��+4HNO3���ҳ��ǵ��أ�B���������ӵõ��ӷ�����ԭ��Ӧ��������������ת�Ƶ�����ȣ����ҳ���B������������5.4gʱ���׳�������������O2�����=
��22.4L/mol=0.28L��
�����ǵ��أ������Ͻ������ӷŵ������������ʣ������Ԫ������Ԫ��֮��D�缫���Ӽ״��缫������D������������ת�Ƶ������֪��������һ�۽���ʱ����Ħ������=
=32g/mol�����Ԫ������Ԫ�أ���Ԫ���Ƿǽ���Ԫ�أ����Դ����������Ƕ��۽�������
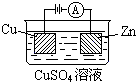
��2=64g/mol�����Ըý�����ͭ������Һ������ͭ��Һ����ѡB��
�ʴ�Ϊ��4AgNO3+2H2O
4Ag+O2��+4HNO3��0.28��B��
��2�����ǵ��أ���Ϊԭ��أ�ͨ��������������A������������Ϊ�����е��������ʴ�Ϊ��������
��3��ȼ�ϵ���У�������ͨ��ȼ�ϣ����������£��״�ˮ�к����������ӷ�Ӧ����̼������Ӻ�ˮ�����Ե缫��ӦʽΪ��CH3OH+8OH--6e-=CO32-+6H2O��
�ʴ�Ϊ��CH3OH+8OH--6e-=CO32-+6H2O��
��4���ҳ��ǵ��أ�̼���������������������Է�Ӧ�ǵ����������Һ����ط�ӦʽΪ��4AgNO3+2H2O
| ||
| ||
| 4 |
�����ǵ��أ������Ͻ������ӷŵ������������ʣ������Ԫ������Ԫ��֮��D�缫���Ӽ״��缫������D������������ת�Ƶ������֪��������һ�۽���ʱ����Ħ������=
| 1.6g | ||
|
| 1.6g | ||
|
�ʴ�Ϊ��4AgNO3+2H2O
| ||
���������⿼����ԭ��غ͵���ԭ������ȷ�ƶ�ԭ������������ǽⱾ��ؼ����Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
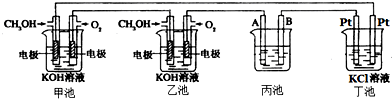
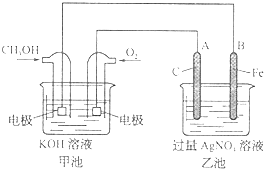 �ݱ�����Ħ��������˾������һ���Լ״�Ϊԭ�ϣ���KOHΪ����ʵ������ֻ��Ŀɳ��ĸ�Чȼ�ϵ�أ���һ�ε������ʹ��һ���£���ͼ��һ���绯ѧ���̵�ʾ��ͼ����֪�׳ص��ܷ�ӦʽΪ��2CH3OH+3O2+4KOH
�ݱ�����Ħ��������˾������һ���Լ״�Ϊԭ�ϣ���KOHΪ����ʵ������ֻ��Ŀɳ��ĸ�Чȼ�ϵ�أ���һ�ε������ʹ��һ���£���ͼ��һ���绯ѧ���̵�ʾ��ͼ����֪�׳ص��ܷ�ӦʽΪ��2CH3OH+3O2+4KOH