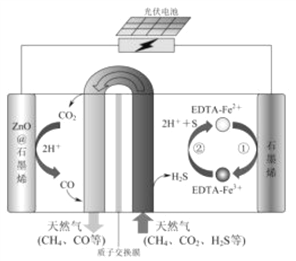
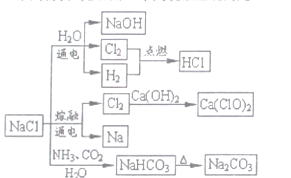
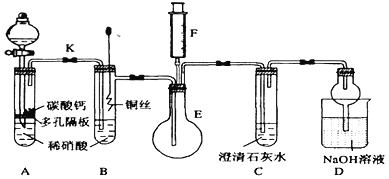
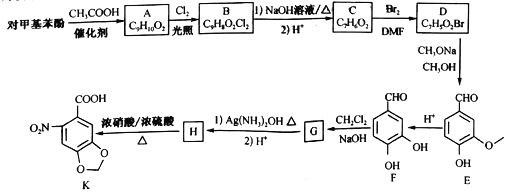
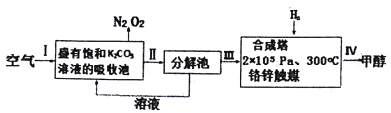
��Ŀ����
����Ŀ�����������ҹ��ĵ��ӹ�ҵѸ�ٷ�չ������˴����ĵ�·��ʴ�̷�Һ�IJ������ŷš�ʴ��Һ��Ҫ�����Ե�(HCl-H2O2)����ͳ��FeCl3��(HCl-FeCl3)�ȷ�����ʴ�̷�Һ�к��д�����Cu2+����Һ�Ļ������ÿɼ���ͭ��Դ����ʧ������ʴ�̷�Һ�ij��ô����������£�
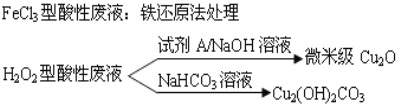
��1��FeCl3�����Է�Һ�û�ԭ������������Fe��Cl2�ֱ���Ϊ��ԭ�������������ɻ���ͭ��ʹʴ��Һ��������������Ҫ��ѧ��Ӧ�У�Fe+Cu2+=Fe2++Cu��Fe+2H+=Fe2++H2��������_______________��__________________��(�����ӷ���ʽ��ʾ)��
��2��HCl-H2O2��ʴ��Һʴ�̹����з����Ļ�ѧ��Ӧ�û�ѧ����ʽ�ɱ�ʾΪ��__________��
��3������H2O2�����Է�Һ����Cu2(OH)2CO3�Ĺ���������Ʒ�Ӧ���¶ȣ����¶ȸ���80��ʱ����Ʒ��ɫ��������ԭ�������____________________________________��
���𰸡�Fe+2Fe3+=3Fe2+2Fe2++Cl2=2Fe3++2Cl-Cu+2HCl+H2O2=CuCl2+2H2O�¶ȸ�ʹ���ﲿ�ַֽ������ɫ��CuO�����²�Ʒ��ɫ����
��������
��1��FeCl3�����Է�Һ�к���Fe3����Fe2����Fe3�����������ԣ�����Fe��Ӧ��Fe2�����л�ԭ�ԣ�����Cl2��Ӧ����2��H2O2�����������¾���ǿ�����ԣ�������Cu����CuCl2����3��Cu2��OH��2CO3�ֽ����ɺ�ɫCuO��
��1��FeCl3�����Է�Һ�к���Fe3����Fe2����Fe3�����������ԣ�����Fe��Ӧ�����ӷ���ʽΪFe+2Fe3��=3Fe2����Fe2�����л�ԭ�ԣ�����Cl2��Ӧ�����ӷ���ʽΪ2Fe2��+Cl2=2Fe3��+2Cl������2��H2O2�����������¾���ǿ�����ԣ�������Cu����CuCl2����Ӧ�Ļ�ѧ����ʽΪCu+2HCl+H2O2=CuCl2+2H2O��
��3�����¶ȸ���80��ʱ��Cu2��OH��2CO3�ֽ����ɺ�ɫCuO�����²�Ʒ��ɫ������