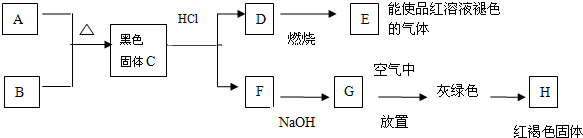
��Ŀ����
8����֪�����������ȵ�����ͭ��Ӧ�õ������ͽ���ͭ����ʾ��ͼ�е�װ�ÿ���ʵ�ָ÷�Ӧ���ش��������⣺��1��A�м�������ʵĻ�ѧʽ�����ǹ���NH4Cl��Ca��OH��2��A�з�����Ӧ�Ļ�ѧ����ʽ��2NH4Cl+Ca��OH��2$\frac{\underline{\;\;��\;\;}}{\;}$CaCl2+2NH3��+2H2O��
��2��B�м���������Ǽ�ʯ�ң��������dz�ȥ�����е�ˮ������
��3��ʵ��ʱ��C�й۲쵽�������Ǻ�ɫ��ĩ��Ϊ��ɫ��������Ӧ�Ļ�ѧ����ʽ��2NH3+3CuO$\frac{\underline{\;\;��\;\;}}{\;}$N2+3H2O+3Cu��
��4��ʵ��ʱ���Թ�D�г�������ɫҺ�壬����ɫҺ�������Ӧ���ǰ�ˮ��
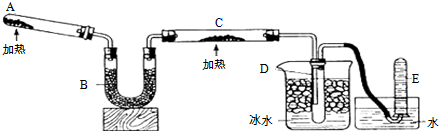
���� ��1������ʵ��Ŀ�ģ��������ȵ�����ͭ��Ӧ�õ������ͽ���ͭ����֪A�м�������Ȼ�狀��������ƵĹ��������������ȡ������������Ӧ�Ļ�ѧ����ʽ�ǣ�2NH4Cl+Ca��OH��2$\frac{\underline{\;\;��\;\;}}{\;}$CaCl2+NH3��+2H2O��
��2���Ƶõİ����к�������ˮ�������ü�ʯ�������գ�
��3������������ͭ�ڼ��ȵ������·�����Ӧ���ɽ���ͭ��ˮ�Լ���������ɫ��ĩ��Ϊ��ɫ��
��4��������ԭ����֮ͭ��ʣ��İ����Ͳ�����ˮ����������ʱ���γɰ�ˮ��
��� �⣺��1������ʵ��Ŀ�ģ��������ȵ�����ͭ��Ӧ�õ������ͽ���ͭ����֪A�м�������Ȼ�狀��������ƵĹ��������������ȡ������������Ӧ�Ļ�ѧ����ʽ�ǣ�2NH4Cl+Ca��OH��2$\frac{\underline{\;\;��\;\;}}{\;}$CaCl2+NH3��+2H2O���ʴ�Ϊ������NH4Cl��Ca��OH��2��2NH4Cl+Ca��OH��2$\frac{\underline{\;\;��\;\;}}{\;}$CaCl2+2NH3��+2H2O��
��2���Ƶõİ����к�������ˮ�������ü�ʯ�������գ��ʴ�Ϊ����ʯ�ң���ȥ�����е�ˮ������
��3������������ͭ�ڼ��ȵ������·�����Ӧ���ɽ���ͭ��ˮ�Լ���������ɫ��ĩ��Ϊ��ɫ��
�ʴ�Ϊ����ɫ��ĩ��Ϊ��ɫ��2NH3+3CuO$\frac{\underline{\;\;��\;\;}}{\;}$N2+3H2O+3Cu��
��4��������ԭ����֮ͭ��ʣ��İ����Ͳ�����ˮ����������ʱ���γɰ�ˮ���ʴ�Ϊ����ˮ��
���� ���⿼��ѧ��������ʵ�����Ʒ��������Լ������Ļ�ԭ�Է����֪ʶ�����ʵ�������飬�������Ѷȣ�

| A�� | 0.5molH2O | B�� | 5.6L���� | C�� | 3.01��1023��N2 | D�� | 44gCO2 |
| A�� | O2��O3 | B�� | ${\;}_{92}^{235}$U��${\;}_{92}^{238}$U | ||
| C�� | ${\;}_1^2$H2��H2 | D�� | ${\;}_1^2$H2O��H2O |
2H2��g��+O2��g���T2H2O��g����H2=-Q2 KJ/mol
2H2��g��+O2��g���T2H2O��1����H3=-Q3KJ/mol
�����£�ȡ�����Ϊ4��1�ļ���������Ļ������11.2L�����ۺϳɱ�״����������ȫȼ�պ�ָ������£�������˵����ȷ���ǣ�������
| A�� | �ų�������Ϊ��0.4Q1+0.1Q3��KJ | B�� | �ų�������Ϊ��0.4Q1+0.05Q2��KJ | ||
| C�� | ��H2����H3 | D�� | ��H2����H3 |
| A�� | ��̼�������Һ�м����������������Һ��Ca2++2HCO3-+2OH-=CaCO3��+2H2O+CO32- | |
| B�� | ��������������������Һ��Al+2OH-=AlO2-+H2�� | |
| C�� | ������������Һ���ն�����̼��2OH-+CO2=CO32-+H2O | |
| D�� | Mg��HCO3��2 ��Һ ����� NaOH ��Һ��Ӧ��Mg2++2HCO3-+2OH-=MgCO3��+CO32-+2H2O |
��±$��_{������}^{������ʯ�ҽ�}$Mg��OH��2$\stackrel{��������}{��}$MgCl2��Һ$\stackrel{������}{��}$MgCl2•6H2O$��_{������}^{HCl����}$MgCl2$��_{������}^{���}$Mg��
| A�� | �������з����ķ�ӦΪ��������ԭ��Ӧ | |
| B�� | �������Ʊ������У�δ����������ԭ��Ӧ | |
| C�� | �������ǹ��� | |
| D�� | ������������Ũ������ȴ�ᾧ |
| A�� | ���ӵ��뺣�����γ�ɳ�� | |
| B�� | ˮ�೧��ұ���ø�ѹ����г��� | |
| C�� | ҽԺ�����˥���߽���ѪҺ�� | |
| D�� | �Ȼ�����Һ�м�����������Һ������ɫ���� |