��Ŀ����
1��ʵ��������NaOH��������2.0mol/L��NaOH��Һ480mL��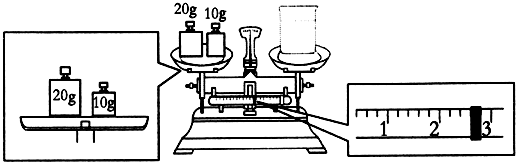
��1������ʱ������ʹ�õIJ����������ձ�������������ͷ�ιܡ�500ml����ƿ
��2��Ҫ��ɱ�ʵ���ͬѧӦ�Ƴ�NaOH40.0 g
��3��ijͬѧ������NaOH��������������������ƽ�����ձ�����������ƽƽ����״̬��ͼ���ձ���ʵ������Ϊ27.4g
��4��ʹ������ƿǰ������е�һ�������Ǽ������ƿ�Ƿ�©ˮ
��5�������ƹ�������������������ȷ�ģ����в������������ƫ�ߵ���B��
A��û��ϴ���ձ��Ͳ����� B��δ��ȴ�����¾ͽ���Һת�Ƶ�����ƿ������
C������ƿ�����������������ˮ
D�����ݺ�����ƿ������ҡ�ȣ����ú�Һ����ڿ̶��ߣ��ټ�ˮ���̶��ߣ�
���� ��1����������һ�����ʵ���Ũ����Һ�IJ���ѡ����Ҫ��������
��2������������Һ���ѡ����ʵ�����ƿ������m=CVM������Ҫ���ʵ�������
��3�����������������ҩƷʱ��ҩƷ��ʵ������=���������-�����������
��4������ƿʹ�ù�������Ҫ���µߵ���ҡ����
��5�������������������ʵ����ʵ�������Һ�����Ӱ�죬����C=$\frac{n}{V}$������������
��� �⣺��1������һ�����ʵ���Ũ����Һ��һ�㲽�裺���㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ������õ��������У���ƽ��ҩ�ס��ձ�����������500mL����ƿ����ͷ�ιܣ�
�����õ��IJ�������Ϊ���ձ�����������500mL����ƿ����ͷ�ιܣ�
�ʴ�Ϊ���ձ�����������500mL����ƿ����ͷ�ιܣ�
��2����NaOH��������2.0mol/L��NaOH��Һ480mL��Ӧѡ��500mL����ƿ����Ҫ�������Ƶ�����m=2.0mol/L��0.5L��40g/mol=40.0g��
�ʴ�Ϊ��40.0��
��3��ͼ�����ʺ������λ�÷ŷ��ˣ�������ϵӦΪ�����������=�ձ�������+������ʾ����������30 g=�ձ�������+2.6 g�����ձ�������Ϊ��30g-2.6g=27.4g��
�ʴ�Ϊ��27.4��
��4������ƿʹ�ù�������Ҫ���µߵ���ҡ����������ʹ��ǰ�������Ƿ�©ˮ��
�ʴ�Ϊ���������ƿ�Ƿ�©ˮ��
��5��A��û��ϴ���ձ��Ͳ��������������ʵ����ʵ���ƫС����ҺŨ��ƫ�ͣ���A����
B��δ��ȴ�����¾ͽ���Һת�Ƶ�����ƿ�����ݣ���ȴ����Һ���ƫС����ҺŨ��ƫ�ߣ���B��ȷ��
C������ƿ�����������������ˮ�������ʵ����ʵ�������Һ��������������Ӱ�죬��ҺŨ�Ȳ��䣬��C����
D�����ݺ�����ƿ������ҡ�ȣ����ú�Һ����ڿ̶��ߣ��ټ�ˮ���̶ȣ�������Һ���ƫ����ҺŨ��ƫ�ͣ���D����
��ѡ��B��
���� ���⿼����һ�����ʵ���Ũ����Һ�����ƣ���Ϥ����ԭ�������ǽ���ؼ���ע�������������ͼ��ɣ���Ŀ�ѶȲ���

 �»����ܶ�Ա��ϵ�д�
�»����ܶ�Ա��ϵ�д� ����ͼ����ּ��������ҵ֣�ݴ�ѧ������ϵ�д�
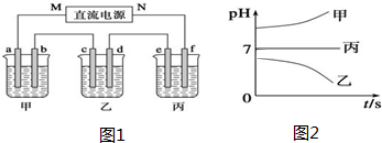
����ͼ����ּ��������ҵ֣�ݴ�ѧ������ϵ�д���ͼ1��ʾװ���У��ס��ҡ��������ձ����ηֱ�ʢ��250mL������X��Һ��������Y��Һ��������Z��Һ���缫��Ϊʯī�缫����ͨ��Դ������һ��ʱ��������c�缫��������6.4g�������¸��ձ�����Һ��pH����ʱ��t�Ĺ�ϵͼ2���£��ݴ˻ش��������⣺
| ������ | Na+��K+��Cu2+ |
| ������ | SO42-��OH- |
��2�����ձ��е缫b�Ϸ����ĵ缫��ӦΪ4OH--4e-=O2��+2H2O��
��3�����ձ������ܷ�Ӧ���ӷ���ʽΪ2Cu2++2H2O$\frac{\underline{\;ͨ��\;}}{\;}$2Cu+O2��+4H+�����ձ���a��b������������״����3.36L�����壮
| A�� | Na2CO3 | B�� | NaHCO3 | ||
| C�� | Na2CO3��NaHCO3 | D�� | NaOH��Na2CO3��NaHCO3 |
| A�� | NH3 | B�� | CH4 | C�� | NaHCO3 | D�� | HNO3 |
| ѡ�� | ʵ����������� | ʵ����� |
| A | ��ij�����м���ϡ���ᣬ������ʹ����ʯ��ˮ����ǵ����� | ˵������һ����̼���� |
| B | ��ij��ɫ��Һ�еμ�BaCl2��Һ���ٵμӹ�����ϡHNO3��������ɫ���� | ����ɫ��Һ��һ����SO42- |
| C | ��ij��ɫ��Һ�еμ�NaOH��Һ�����Ⱥ�����ʪ��ĺ�ɫʯ����ֽ���������� | ˵����ҺҺ�к���NH4+ |
| D | ij��ɫ����ͨ�����ȵ�CuO��CuO��Ϊ��ɫ | ������һ��Ϊ���� |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | 0.3 mol•L-1 K2SO4��Һ�к���0.6NA��K+��0.3 mol��SO42- | |
| B�� | �ڱ�״����H2O��Ħ�����Լ��22.4 L•mol-1 | |
| C�� | ��58.5g��NaCl�����ܽ���1L��ˮ�У����õ���Һ���ʵ���Ũ��Ϊ1mol/L | |
| D�� | 1 mol�κ������ڱ�״���µ������ԼΪ22.4 L |

 ��1��ij���淴Ӧ��ABC��Ϊ���壩��0-2���ӽ��й����У��ڲ�ͬ��Ӧʱ������ʵ����ı仯�����ͼ��ʾ��
��1��ij���淴Ӧ��ABC��Ϊ���壩��0-2���ӽ��й����У��ڲ�ͬ��Ӧʱ������ʵ����ı仯�����ͼ��ʾ��