��Ŀ����
(10��)��������HA��������NaA�Ļ����Һ���ڻ�ѧ������������Һ�������м�����������ʱ����Һ������Ա仯����
(1)�����Һ�м�����������ʱ��������Ӧ�����ӷ���ʽ�����������������������м�������KOH��Һʱ��������Ӧ�����ӷ���ʽ������������������������
(2)�ֽ�0��04 mol��L-1HA��Һ��0��02 mol��L-1NaOH��Һ�������ϣ��õ�������Һ��
����HAΪHCN������Һ�Լ��ԣ�����Һ��c(Na��)����c(��N��)(�<������������>��)����ó��ý��۵�������������������������������������
����HAΪCH��COOH������Һ�����ԡ���Һ�����е����Ӱ�Ũ���ɴ�С���е�˳����������������������������������������������������������
���𰸡�
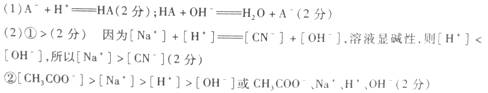
��������

��ϰ��ϵ�д�
�����Ŀ