��Ŀ����
��1��Ԫ��M�Ƕ�����Ԫ�أ��䳣�������ں�ˮ�У����ʱ���Ϊ��������������
��M��ԭ�ӽṹʾ��ͼΪ______��
����M��AlΪ�缫��KOH��ҺΪ�������Һ�����ĵ缫��ӦʽΪ______��
��2������ǽ������������ȵ�ij�¶ȣ��漴�����������п�����ȴ�Ľ����ȴ������ա�
��ʹ��ˮ���д�������ɴ������������÷�Ӧ�Ļ�ѧ����ʽΪ____________
����֤����ˮ����Ĺ�������Ƿ����+3�۵�������ѡ�õ��Լ�Ϊ_______ (�����)
��3���{�������ˮ�еķ�ӦΪ4FeO42��+10H2O 4Fe(OH)3+8OH��+3O2
4Fe(OH)3+8OH��+3O2
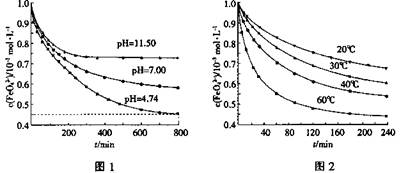
��ͼ1��25��ʱK2FeO4�ڲ�ͬpH��Һ��Ũ�ȵı仯�����pH =4.74ʱ����Ӧ�ӿ�ʼ��800min��ƽ����Ӧ����v(FeO42��)=______ (������λ��Ч���֣���
��ͼ1��800min��������Һ��K2FeO4��Ũ�Ⱦ����ٸı䡣�۲�ͼ1�ж�����pH ��˷�Ӧ��ƽ�ⳣ��______����������С�����䡱����
��ͼ2��240min��������Һ��K2FeO4��Ũ�Ⱦ����ٸı䣬��������Ӧ�ķ�Ӧ�ȡ�H______0(�>������<������=������
��M��ԭ�ӽṹʾ��ͼΪ______��
����M��AlΪ�缫��KOH��ҺΪ�������Һ�����ĵ缫��ӦʽΪ______��
��2������ǽ������������ȵ�ij�¶ȣ��漴�����������п�����ȴ�Ľ����ȴ������ա�
��ʹ��ˮ���д�������ɴ������������÷�Ӧ�Ļ�ѧ����ʽΪ____________
����֤����ˮ����Ĺ�������Ƿ����+3�۵�������ѡ�õ��Լ�Ϊ_______ (�����)
| A��H2O2��Һ | B��ͭ�� | C��ϡ���� | D��KMnO4��Һ |
 4Fe(OH)3+8OH��+3O2
4Fe(OH)3+8OH��+3O2��ͼ1��25��ʱK2FeO4�ڲ�ͬpH��Һ��Ũ�ȵı仯�����pH =4.74ʱ����Ӧ�ӿ�ʼ��800min��ƽ����Ӧ����v(FeO42��)=______ (������λ��Ч���֣���
��ͼ1��800min��������Һ��K2FeO4��Ũ�Ⱦ����ٸı䡣�۲�ͼ1�ж�����pH ��˷�Ӧ��ƽ�ⳣ��______����������С�����䡱����
��ͼ2��240min��������Һ��K2FeO4��Ũ�Ⱦ����ٸı䣬��������Ӧ�ķ�Ӧ�ȡ�H______0(�>������<������=������

��15�֣���1���� ��2�֣�
��2�֣�
��Al+4OH����3e��=[Al(OH)4]����AlO2��+2H2O��2�֣�
��2����3Fe+4H2O Fe3O4+4H2����3�֣�
Fe3O4+4H2����3�֣�
��BC��2�֣�
��3����6.9��10��7mol?L��1?mol��1��2�֣�
�ڲ��䣨2�֣�
��>��2�֣�
 ��2�֣�
��2�֣� ��Al+4OH����3e��=[Al(OH)4]����AlO2��+2H2O��2�֣�
��2����3Fe+4H2O
 Fe3O4+4H2����3�֣�
Fe3O4+4H2����3�֣���BC��2�֣�
��3����6.9��10��7mol?L��1?mol��1��2�֣�
�ڲ��䣨2�֣�
��>��2�֣�
�����������1���ٸ��������֪MΪþ���˵����Ϊ+12��������Ӳ�ṹΪ282����þ������KOH��Һ����������KOH��Һ������������������������Ӧ����ӦʽΪAl+4OH����3e��=[Al(OH)4]����AlO2��+2H2O����2���ٺ��ȵ�������ˮ��Ӧ��������������������������3Fe+4H2O
 Fe3O4+4H2������Fe3O4�Ǽ��������������ϡ���ᣬ����ˮ��Fe3+��Fe2+��˫��ˮ������ؾ�������Fe2+��ͭ���ܻ�ԭFe3+��Fe3+������ͭ����BC��ȷ����3���ٶ�ͼ��֪����c(FeO42��)=(1.0��0.45)��10��3mol/L���ɡ�c/��t��֪��v(FeO42��)=(1.0��0.45)��10��3mol/L��800min=6.867��10��7mol/(L?min)��6.9��10��7mol/(L?min)��������pH����¶Ȳ��䣬��ƽ�ⳣ�����䣻�������¶����ߣ�c(FeO42��)��С����ƽ�����ƣ�˵������Ӧ�����ȷ�Ӧ�����H>0��
Fe3O4+4H2������Fe3O4�Ǽ��������������ϡ���ᣬ����ˮ��Fe3+��Fe2+��˫��ˮ������ؾ�������Fe2+��ͭ���ܻ�ԭFe3+��Fe3+������ͭ����BC��ȷ����3���ٶ�ͼ��֪����c(FeO42��)=(1.0��0.45)��10��3mol/L���ɡ�c/��t��֪��v(FeO42��)=(1.0��0.45)��10��3mol/L��800min=6.867��10��7mol/(L?min)��6.9��10��7mol/(L?min)��������pH����¶Ȳ��䣬��ƽ�ⳣ�����䣻�������¶����ߣ�c(FeO42��)��С����ƽ�����ƣ�˵������Ӧ�����ȷ�Ӧ�����H>0��
��ϰ��ϵ�д�
�����Ŀ
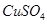 ��Һ������ͼʾ��ʾ����
��Һ������ͼʾ��ʾ����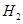 �����(V)��ʱ��(t)�Ĺ�ϵ��������ȷ����
�����(V)��ʱ��(t)�Ĺ�ϵ��������ȷ����