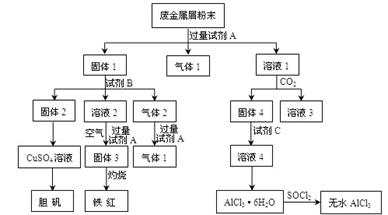
��Ŀ����
��15�֣�ij��ѧ��ȤС���ú�����������ͭ�ĺϽ���ȡ�������Ȼ�����Һ���̷�����͵������壬��̽����ҵ���ϵ������á���ʵ�鷽�����£�
��ش���������:
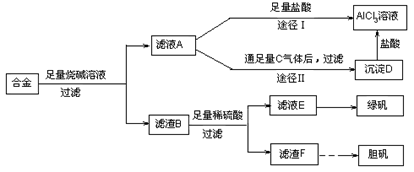
��1������ҺA�Ƶ�AlCl3��Һ��;����͢�����������Ϊ�������� ��������:
��
��2������ҺE�еõ��̷������ʵ������� ��
��3������һ���ܷ�Ӧʽ��ʾ������F�Ʊ�������������з����ı仯:
��
��4����ͬѧ����ɽ�����������ܽ�Ͻ���ռ�������ᣬ������Ʒ�����Ҳ���Ƶ��������ʣ�����Ϊ���ߵķ����Ƿ������ ��������
��

��ش���������:
��1������ҺA�Ƶ�AlCl3��Һ��;����͢�����������Ϊ�������� ��������:
��
��2������ҺE�еõ��̷������ʵ������� ��
��3������һ���ܷ�Ӧʽ��ʾ������F�Ʊ�������������з����ı仯:
��
��4����ͬѧ����ɽ�����������ܽ�Ͻ���ռ�������ᣬ������Ʒ�����Ҳ���Ƶ��������ʣ�����Ϊ���ߵķ����Ƿ������ ��������
��
��1��;�������������Ϊ��ҺA��NaAlO2��Һ����;��Iֱ����A��������õ���AlCl3��Һ�к��д�����NaCl���ʣ���;��II��ͨ��c��CO2�����壬��D[Al(OH)3]��������Al(OH)3�ܽ��������еõ����Ǵ�����AlCl3��Һ������;��II��������2������Ũ������ȴ�ᾧ��ϴ�ӡ����3��2Cu + O2 + 2H2SO4+8H2O 2CuSO4��5H2O ��4�����ߵķ��������� ��Ϊ�÷���������ʵ�鷽����Ƶļ�Լ��ԭ����������ࡢ�����Լ�������ʱ�䳤��
2CuSO4��5H2O ��4�����ߵķ��������� ��Ϊ�÷���������ʵ�鷽����Ƶļ�Լ��ԭ����������ࡢ�����Լ�������ʱ�䳤��
 2CuSO4��5H2O ��4�����ߵķ��������� ��Ϊ�÷���������ʵ�鷽����Ƶļ�Լ��ԭ����������ࡢ�����Լ�������ʱ�䳤��
2CuSO4��5H2O ��4�����ߵķ��������� ��Ϊ�÷���������ʵ�鷽����Ƶļ�Լ��ԭ����������ࡢ�����Լ�������ʱ�䳤�������������1��ƫ�����ƺ��������ᷴӦ�����Ȼ������Ȼ��ƵĻ�������ƫ��������ͨ�������Ķ�����̼����õ������������������������������ܽ⣬������Һ�ijɷ�ֻ���Ȼ������ʴ�Ϊ������Ϊ��ҺA��ƫ�����ƺ�����������Һ����;��Iֱ����A�м�������õ���AlCl3��Һ�к��д������Ȼ������ʣ���;��IIͨ�������̼���壬��Al(OH)3��������Al(OH)3�ܽ��������еõ����Ǵ�����AlCl3��Һ����2�������Һ���Ʊ��̷�ʱӦ�Ƚ���Һ����Ũ����Ȼ����ȴ�ᾧ��ϴ�Ӹ���ɵõ��̷����ʴ�Ϊ������Ũ������ȴ�ᾧ��ϴ�ӡ������3���ɿ����Ʊ��������ʿ���Ϊͭ��ͭ���ܺ�ϡ���ᷴӦ�������Ժ����ᷴӦ����������F�ǽ���ͭ��Cu�м���ϡ��������Ʊ��������壬��Ӧ����ʽΪ��2Cu+2H2SO4+8H2O+O2=2CuSO4?5H2O����4����ʵ�鷽��һ�У�������Ŀ�����û�й��ߵ�Ҫ��ֻ��������ɣ����Ƿ������У���Ҫ�����������ᣬ����ѿأ��ʴ�Ϊ������������Ϊ�÷�����������ࡢʱ�䳤�������Լ�����

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
 4Fe(OH)3+8OH��+3O2
4Fe(OH)3+8OH��+3O2