��Ŀ����
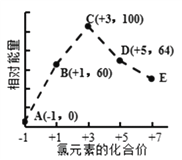
����Ŀ��һ���ܱ��������м���һ�����ɻ����ĸ��壨��Ȳ��ƣ��������ֳ������֣�����߳���8molN2���ұ߳���CO��CO2�Ļ�����干64gʱ�����崦����ͼλ�ã������¶Ȳ��䣩������˵����ȷ����( )

A.�ұ�CO��CO2������֮��Ϊ1:3
B.�ұ�CO������Ϊ42g
C.�ұ������ܶ�����ͬ�����������ܶȵ�2��
D.���ı��ұ�CO��CO2�ij�������ʹ���崦�ھ����Ҷ�![]() �����������¶Ȳ��䣬��ǰ�����������ڵ�ѹǿ֮��Ϊ5:6
�����������¶Ȳ��䣬��ǰ�����������ڵ�ѹǿ֮��Ϊ5:6
���𰸡�BD
��������
�������ð����ӵ����ɺͰ����ӵ������۽��з������������������¶ȡ�ѹǿ��ͬ����ͬ�����£����֮�ȵ������ʵ���֮�ȣ��������֮��Ϊ4��1���������������ʵ���֮��Ϊ4��1�������Ҳ��������ʵ��� =2mol��CO��CO2����Ϊ64g����CO�����ʵ���Ϊxmol���������̼���ʵ���Ϊ(2-x)mol��28xg+44(2-x)g=64g��x=1.5mol����CO�����ʵ���Ϊ1.5mol��������̼���ʵ���Ϊ0.5mol���ɴ˷������
A�����岻����˵������ѹǿ��ͬ���¶���ͬ��ѹǿ��ͬ������ȵ������ʵ���֮�ȣ������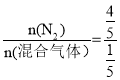 ��������ֵ���ó�n(�������)=2mol�������n(CO)��n(CO2)=2mol��28n(CO)��44n(CO2)=64g��n(CO2)=0.5mol��n(CO)=1.5mol��n(CO)��n(CO2)=1.5��0.5=3��1��CO��CO2������֮�ȵ������ʵ���֮�ȣ���CO��CO2�ķ�����֮�ȵ���3��1����A����
��������ֵ���ó�n(�������)=2mol�������n(CO)��n(CO2)=2mol��28n(CO)��44n(CO2)=64g��n(CO2)=0.5mol��n(CO)=1.5mol��n(CO)��n(CO2)=1.5��0.5=3��1��CO��CO2������֮�ȵ������ʵ���֮�ȣ���CO��CO2�ķ�����֮�ȵ���3��1����A����
B������Aѡ�������CO������Ϊ1.5mol��28g��mol��1=42g����B��ȷ��
C����ͬ�����£������ܶȵ�֮�ȵ���Ħ������֮�ȣ���������Ħ������Ϊ![]() =32g��mol��1����˻��������ܶ�����ͬ�����������ܶ���ȣ���C����
=32g��mol��1����˻��������ܶ�����ͬ�����������ܶ���ȣ���C����
D����������Ҷ�![]() �������ҿռ����֮��Ϊ2��1������CO2��CO�����ʵ���Ϊ4mol����ͬ��������������ʵ���֮�ȵ�����ѹǿ֮�ȣ�������ѹǿ֮��Ϊ(8+2)mol��(8+4)mol=5��6����D��ȷ��
�������ҿռ����֮��Ϊ2��1������CO2��CO�����ʵ���Ϊ4mol����ͬ��������������ʵ���֮�ȵ�����ѹǿ֮�ȣ�������ѹǿ֮��Ϊ(8+2)mol��(8+4)mol=5��6����D��ȷ��
��ΪBD��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ���л�������ʷ�ƾã��ں�嫵���ʷ��������������ڻ�ѧ�ļ��أ�����˵������������(����)
ѡ�� | �Ŵ����� | �������� | �漰ԭ�� |
A | ������������� | �����������Ϊͭ�� | ���ý����û������ý��� |
B | ��������¶��һǮն���� | ��һ��һǮ��ǧ��ǧǮ������ľ�ϣ�ˮ��ʯ���� | ���漰��ѧ�仯 |
C | ���칤��� | ������ҩ����Ϊ��������Ϊ������ | ����ָ���ϼ۽��ͣ���������ص������� |
D | ��������� | ������һ�գ���ˮ�����գ���ȡ֭�� | ��������������ȡ������ |
A. AB. BC. CD. D
����Ŀ����1��25 ��ʱ���Ʊ������������漰���Ȼ�ѧ����ʽ��ƽ�ⳣ�������
�Ȼ�ѧ����ʽ | ƽ�ⳣ�� | |
�� | 2NO2(g)+NaCl(s) | K1 |
�� | 4NO2(g)+2NaCl(s) | K2 |
�� | 2NO(g)+Cl2(g) | K3 |
����¶��£���H3=_______________kJmol-1��K3=_____________����K1��K2��ʾ����
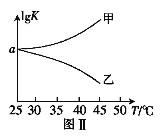
��2��25��ʱ�������Ϊ2L�ĺ����ܱ�������ͨ��0.08 mol NO��0.04 molCl2����������Ӧ�ۣ�����Ӧ��ʼ�����ʱ�¶���ͬ������ѹǿ����ʾ��Ӧ������ѹǿ(p)��ʱ��(t)�ı仯��ͼ��ʵ����ʾ����H3 ___���>����<����=����0��������������ͬ�����ı�ijһ�����������ѹǿ��ʱ��ı仯��ͼ��������ʾ����ı��������_____________����5 minʱ���ٳ���0.08 mol NO��0.04 molCl2�����������ƽ����Է���������_____________�����������С�����䡱����ͼ���Ǽס�����ͬѧ���������Ӧ�۵�ƽ�ⳣ���Ķ���ֵ��lgK�����¶ȵı仯��ϵͼ��������ȷ��������______����ס����ҡ�����aֵΪ__________��25 ��ʱ��÷�Ӧ����ijʱ�̣�NO(g)��Cl2(g)��NOCl(g)��Ũ�ȷֱ�Ϊ0.8��0.1��0.3�����ʱv��_________v�����>����������=����

(3)��300 �桢8 MPa�£���CO2��H2�����ʵ���֮��1��3 ͨ��һ�ܱ������з���CO2(g)��3H2(g)![]() CH3OH(g)��H2O(g)�з�Ӧ���ﵽƽ��ʱ�����CO2��ƽ��ת����Ϊ50%����÷�Ӧ�����µ�ƽ�ⳣ��ΪKp��_____(��ƽ���ѹ����ƽ��Ũ�ȼ��㣬��ѹ����ѹ�����ʵ�������)��
CH3OH(g)��H2O(g)�з�Ӧ���ﵽƽ��ʱ�����CO2��ƽ��ת����Ϊ50%����÷�Ӧ�����µ�ƽ�ⳣ��ΪKp��_____(��ƽ���ѹ����ƽ��Ũ�ȼ��㣬��ѹ����ѹ�����ʵ�������)��