��Ŀ����
�ֶ�0.1 mol/L�Ĵ�����Һ��������о���
��1����pH��ֽ�ⶨ����Һ��pH������ȷ�IJ���
��
��2��������Һ�ʼ��Ե�ԭ����(�����ӷ���ʽ��ʾ) ��
��3��ijͬѧ��Ϊ����Һ��Na2CO3��ˮ�������ģ�����ˮ���CO32�����Ӳ�������������10�����������ʵ��֤����ͬѧ�Ĺ۵��Ƿ���ȷ��
��
��4��ijͬѧ������ѧ֪ʶ����Һ���з� ����д���������ĸ���ϵʽ�������жϣ�����ȷ�Ĺ�ϵʽ�����
����д���������ĸ���ϵʽ�������жϣ�����ȷ�Ĺ�ϵʽ����� �����ڴ���ĺ���д����ȷ�Ĺ�ϵʽ��
�����ڴ���ĺ���д����ȷ�Ĺ�ϵʽ��
�� c()�� 2[c(2��3 )��c(HCO��3 ) ]�� ��
�� c()��c(H+)�� 2 c(2��3 )��c(HCO��3 )��c(OH��)�� ��
�� c(OH��)�� c(H+)��c(HCO��3 ) ��c()�� ��
�� c()��c(2��3 )��c(OH��)��c(HCO��3 ) �� ��
��1����pH��ֽ�ⶨ����Һ��pH������ȷ�IJ���
��
��2��������Һ�ʼ��Ե�ԭ����(�����ӷ���ʽ��ʾ) ��
��3��ijͬѧ��Ϊ����Һ��Na2CO3��ˮ�������ģ�����ˮ���CO32�����Ӳ�������������10�����������ʵ��֤����ͬѧ�Ĺ۵��Ƿ���ȷ��
��
��4��ijͬѧ������ѧ֪ʶ����Һ���з�
 ����д���������ĸ���ϵʽ�������жϣ�����ȷ�Ĺ�ϵʽ�����
����д���������ĸ���ϵʽ�������жϣ�����ȷ�Ĺ�ϵʽ����� �����ڴ���ĺ���д����ȷ�Ĺ�ϵʽ��
�����ڴ���ĺ���д����ȷ�Ĺ�ϵʽ���� c()�� 2[c(2��3 )��c(HCO��3 ) ]�� ��
�� c()��c(H+)�� 2 c(2��3 )��c(HCO��3 )��c(OH��)�� ��
�� c(OH��)�� c(H+)��c(HCO��3 ) ��c()�� ��
�� c()��c(2��3 )��c(OH��)��c(HCO��3 ) �� ��
��1����С��pH��ֽ���ڱ�������Ƭ���ϣ��ò�����պȡ����Һ������ֽ���в�������ֽ��ɫ�������ɫ���Ա�ȷ����Һ��pH ֵ(2��)
��2��CO32��+H2O HCO3��+OH��(2��)
HCO3��+OH��(2��)
��3����pH��ֽ����pH�ƣ��ⳣ����0.1 mol/L������Һ��pH����pH��12�����ͬѧ�Ĺ۵���ȷ����pH��12�����ͬѧ�Ĺ۵㲻��ȷ��(2��)
��4����c() ="== " 2[c(2��3 )��c(HCO��3 ) ��c()] (1��) �� (1��)
(1��)
��c(OH��) === c(H+)��c(HCO��3 ) ��2c() (1��) �� (1��)
(1��)
��2��CO32��+H2O
 HCO3��+OH��(2��)
HCO3��+OH��(2��)��3����pH��ֽ����pH�ƣ��ⳣ����0.1 mol/L������Һ��pH����pH��12�����ͬѧ�Ĺ۵���ȷ����pH��12�����ͬѧ�Ĺ۵㲻��ȷ��(2��)
��4����c() ="== " 2[c(2��3 )��c(HCO��3 ) ��c()] (1��) ��
 (1��)
(1��)��c(OH��) === c(H+)��c(HCO��3 ) ��2c() (1��) ��
 (1��)
(1��)��

��ϰ��ϵ�д�
�����Ŀ
 ��Ҫ�ɷ�������������������������ˮ������
��Ҫ�ɷ�������������������������ˮ������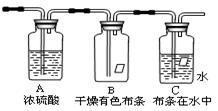
 CO2����2H2O��2SO2��
CO2����2H2O��2SO2��
 ��˳���������������ҵķ����ǣ���װ�õı�ţ���
��˳���������������ҵķ����ǣ���װ�õı�ţ��� �����ܡ���__________��__________��__________��
�����ܡ���__________��__________��__________�� ��������Ϊbg����a��b��ʾCO2��SO2�����ʵ���������������������
��������Ϊbg����a��b��ʾCO2��SO2�����ʵ��������������������� ���ԭϡ��Һ��I-Ũ�ȣ��ζ�ʱ�ķ�ӦΪ��2Na2S2O3 + I2 = Na2S4O6 + 2NaI���Իش�
���ԭϡ��Һ��I-Ũ�ȣ��ζ�ʱ�ķ�ӦΪ��2Na2S2O3 + I2 = Na2S4O6 + 2NaI���Իش� ____________����ǡ����ǡ�������ΪAgX�����������Ļ�ѧʽΪ__________������ΪAgX��������˿ղ����
____________����ǡ����ǡ�������ΪAgX�����������Ļ�ѧʽΪ__________������ΪAgX��������˿ղ����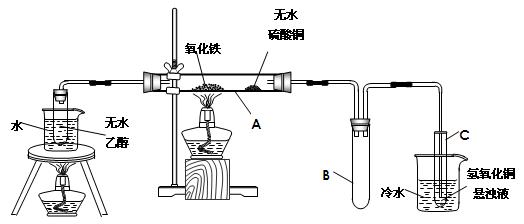

 ����������Һ
����������Һ