��Ŀ����
16��п��һ��Ӧ�ù㷺�Ľ�����Ŀǰ��ҵ����Ҫ���á�ʪ��������ұ��п��ij��п�����Ҫ�ɷ�ΪZnS����������FeS�������ɷ֣�������Ϊԭ��ұ��п�Ĺ���������ͼ��ʾ��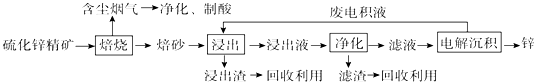
�ش��������⣺
��1����п����ı������������յķ���¯�н��У���������ɰ����Ҫ�ɷֵĻ�ѧʽΪZnO��
��2�����չ����в����ĺ��������ɾ������ᣬ��������ں����Ľ���������
��3������Һ�������������м������Ҫ����ΪZn�ۣ����������û���Fe�ȣ�
��4�������������е������������壬��������Pb-Ag�Ͻ���Ե缫�������ݳ���������O2��
��5���Ľ���пұ�����գ������ˡ���ѹ�������ȫʪ�����̣���ʡ�������¿�����Ⱦ�ı��չ��̣��ֿɻ��һ���й�ҵ��ֵ�ķǽ������ʣ�����ѹ������з�����Ҫ��Ӧ�����ӷ���ʽΪ2ZnS+4H++O2=2Zn2++2S��+2H2O��
��6���ҹ��Ŵ������á�������ұ��п��������Ӧ�����ġ��칤������й��ڡ�������Ǧ���ļ��أ���¯��ʯʮ�װ����һ����ڣ�����Ȼ�������ú̿����ʢ�������н�������Ѻ죬����������ٹ�ȡ������������ǦҲ��������п���չ�����Ҫ��Ӧ�Ļ�ѧ����ʽΪZnCO3+2C$\frac{\underline{\;����\;}}{\;}$Zn+3CO������ע��¯��ʯ����Ҫ�ɷ�Ϊ̼��п����Ǧ��ָ����п��
���� ��п����ı��տ�����ZnO���������ȣ������������к���������������Ʊ����ᣬ����Һ�����������������п�����������������п��ַ�Ӧ�����û���������Һ����Ҫ��������п�������ɵõ�п�����ᣬ���Һ�к������ᣬ��ѭ�����ã�
��1����п����ı������������յķ���¯�з���������ԭ��Ӧ���ݴ��жϱ�ɰ�ɷ֣�
��2���������ɵĺ����������ת��Ϊ���
��3��������֪���ú�п���л�����FeS�����ʣ�ͨ������������ת��Ϊ�������ӣ��ɼ���п�۳�ȥ�������ӣ��Ӷ���ȥFe��
��4�������������е������������壬��������Pb-Ag�Ͻ���Ե缫�������Ϊ����п����������������Ӧ���Դ��ж��������
��5����п��������ѹ�������Ӧ���ɶ���п���ӡ����ˮ��
��6��̼��п��̼�ڸ����·�Ӧ����п��һ����̼�����������ԭ��Ӧ��ʧ�����غ���ƽ����ʽ��
��� �⣺��п����ı��տ�����ZnO���������ȣ������������к���������������Ʊ����ᣬ����Һ�����������������п�����������������п��ַ�Ӧ�����û���������Һ����Ҫ��������п�������ɵõ�п�����ᣬ���Һ�к������ᣬ��ѭ�����ã�
��1����п����ı������������յķ���¯�з���������ԭ��Ӧ����������п����ɰ����Ҫ�ɷ�ΪZnO���ʴ�Ϊ��ZnO��
��2���������ɵĺ����������ת��Ϊ���ᣬ���ں����Ľ����������ʴ�Ϊ��������
��3���ú�п���л�����FeS�����ʣ�����������ת��Ϊ�������ӣ��ɼ���п�۳�ȥ�������ӣ��Ӷ���ȥFe���ʴ�Ϊ��Zn�ۣ��û���Fe�ȣ�
��4�������������е������������壬��������Pb-Ag�Ͻ���Ե缫�������Ϊ����п���������������ӷŵ磬����������Ӧ�����������ʴ�Ϊ��O2��
��5����п��������ѹ�������Ӧ���ɶ���п���ӡ����ˮ����ѧ����ʽΪ��2ZnS+4H++O2=2Zn2++2S��+2H2O���ʴ�Ϊ��2ZnS+4H++O2=2Zn2++2S��+2H2O��
��6��̼��п��̼�ڸ����·�Ӧ����п��һ����̼����ѧ����ʽΪZnCO3+2C$\frac{\underline{\;����\;}}{\;}$Zn+3CO�����ʴ�Ϊ��ZnCO3+2C$\frac{\underline{\;����\;}}{\;}$Zn+3CO����
���� ��������������γɿ������ʵķ��롢�ᴿ��Ϊ�߿��ɳ������ͣ�������ѧ���ķ���������ʵ�������Ŀ��飬ע��������ʵ������Լ�ʵ���ԭ�����ѶȲ���

| A�� | ȼ������101kPaʱ��1mol��������ȫȼ�������ȶ���������ʱ���ų������� | |
| B�� | ��ҵ���õ�ȼH2��Cl2�������ķ����������� | |
| C�� | �������Ż�ʱ���ø����ɳ������� | |
| D�� | ����������й������ǹ����β��� |

����˵������ȷ���ǣ�������
| A�� | �Լ�A������ϡ��������� | |
| B�� | �����Լ�B��ȥMg2+��Fe3+ | |
| C�� | ����1����Ҫ�ɷ���SiO2 | |
| D�� | ���������ʱ�������϶��ڲ���̿�� |
| A�� | NaOH��NaCl | B�� | HCl��Cl2 | C�� | H2SO4��H2 | D�� | H2S��S |
| A�� | MgCl2 | B�� | NaOH | C�� | CaO | D�� | SO2 |
��Ħ���ǹ��ʵ�λ�����߸�����������֮һ����1mol�κ����ʶ�����Լ6.02��1023��ԭ�ӣ���6.02��1023���ǰ����ӵ�����������ԭ�ӵ�Ħ��������1g����HCl��Ħ����������1mol HCl���ӵ���������1mol CO2�к���1mol̼��2mol����
| A�� | �٢ڢ� | B�� | �ڢۢ� | C�� | �ڢܢܢ� | D�� | ȫ�� |
| A�� | ԭ��Һ�п��ܺ���K+��һ��û��HCO3-��OH-������������ɫ����ͨ�����ʯ��ˮ�У�����ʯ��ˮ����� | |
| B�� | ԭ��Һ��һ������Cl-��NO3-�����ܺ���SO42-����Ӧ���ɵ���ɫ���������������ɫ | |
| C�� | ԭ��Һ��������������ΪMg2+��Fe2+������ϡ�����������ķ�ӦΪ3Fe2++4H++NO3-�T3Fe3++NO��+2H2O | |
| D�� | ����ԭ��Һ�м������NaOH��Һ�������ó������ˡ�ϴ�ӡ����յ����أ��õ��Ĺ�������Ϊ107 g |