��Ŀ����
����Ŀ������������һ�ֽྻ������������Դ����������(��Ҫ�ɷ�ΪCO��CO2��)��H2��ϣ����ϳɼ״��������������õķ���֮һ��
(1)������Ӧ�Ĵ�������Cu��Zn��Al��Ԫ�ء�д����̬Znԭ�ӵĺ�������Ų�ʽ��________��
(2)���ݵȵ���ԭ����д��CO���ӵĽṹʽ__________��
(3)�״��������ɵõ���ȩ����ȩ������Cu(OH)2�ļ�����Һ��Ӧ����Cu2O������
�ټ�ȩ������̼ԭ�ӹ�����ӻ�����Ϊ_______________��
�ڼ�ȩ���ӵĿռ乹����___________��1 mol��ȩ��������������ĿΪ______________��
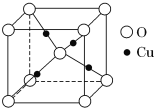
����1��Cu2O����(�ṹ��ͼ��ʾ)�У���������Cuԭ����ĿΪ______________��
���𰸡�1s22s22p63s23p63d104s2��[Ar]3d104s2 C��O sp2�ӻ� ƽ�������� 3NA 4
��������
��1��Znԭ�Ӻ��������Ϊ30�����������Ų�ʽΪ��1s22s22p63s23p63d104s2��
��2�����ݵȵ���ԭ������֪CO��N2Ϊ�ȵ����壬N2���ӵĽṹʽΪN��N����Ϊ�ȵ�������ӵĽṹ���ƣ���CO�ĽṹʽΪC��O��
��3���ټ�ȩ�����к���̼��˫��������3���Ҽ�����̼ԭ�ӹ�����ӻ�����Ϊsp2�ӻ���
�ڼ�ȩ������̼ԭ�ӹ�����ӻ�����Ϊsp2�ӻ���ƽ�������Σ�1mol��ȩ�����Ц� ������ĿΪ2NA��C-H�� ����NA��C-O�Ħ� ��������һ��3NA��
����Cu2O�����ṹ��֪����1��Cu2O�����У��ṹ��ͼ��ʾ������������Cuԭ����ĿΪ4����

 ѧҵ����һ��һ��ϵ�д�
ѧҵ����һ��һ��ϵ�д�����Ŀ��һ���¶���,��3�������Ϊ1.0L�ĺ����ܱ������з�����Ӧ2SO2(g)+O2(g)![]() 2SO3(g)����H<0���ﵽƽ��ʱ,����˵����ȷ����
2SO3(g)����H<0���ﵽƽ��ʱ,����˵����ȷ����
���� | �¶�/�� | ���ʵ���ʼŨ��/mol��L-1 | ���ʵ�ƽ��Ũ��/mol��L-1 | ||
c(SO2) | c(O2) | c(SO3) | c(SO3) | ||
�� | 758 | 0.2 | 0.1 | 0 | 0.044 |
�� | 758 | 0.1 | 0.05 | 0 | |
�� | 858 | 0.2 | 0.1 | 0 | |
A. �ӿ�ʼ��ƽ��ʱ,��������SO3�ķ�Ӧ����Ϊ0.044 mol��L-1��s-1
B. ƽ��ʱ,��������SO3��Ũ��С��0.022mol��L-1
C. ƽ��ʱ,��������SO3��Ũ�ȴ���0.044mol��L-1
D. ����ʼʱ,���������г���0.02mol SO2��0.01mol O2��0.02mol SO3,��Ӧ���淴Ӧ�������