��Ŀ����
�Ȼ�����Ʒ�к�������̼��ء�����غͲ�����ˮ�����ʣ�Ϊ���ᴿ�Ȼ��أ��Ƚ���Ʒ��������ˮ�У���ֽ������ˣ��ٽ���Һ����ͼ��ʾ������в�����
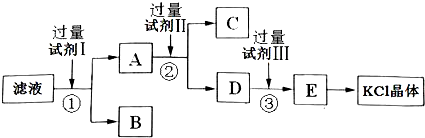
�ش��������⣺
��1��������Һ�е�SO42-�ķ�����
��2���Լ�I�Ļ�ѧʽΪ
��3���Լ���Ļ�ѧʽΪ
��4���Լ����������
��5��ijͬѧ��ȡ�ᴿ�IJ�Ʒ0.745g���ܽ������100mL����ƿ�У�ÿ��ȡ25.00mL��Һ����0.1000mol?L-1������������Һ��Ӧ�����η�Ӧ��������������Һ��ƽ�����Ϊ23.50mL���ò�Ʒ�Ĵ���Ϊ

�ش��������⣺
��1��������Һ�е�SO42-�ķ�����
ȡ������Һ���Թ��У������м��������ữ��BaCl2��Һ�����а�ɫ�������ɣ�����SO42-����֮����
ȡ������Һ���Թ��У������м��������ữ��BaCl2��Һ�����а�ɫ�������ɣ�����SO42-����֮����
����2���Լ�I�Ļ�ѧʽΪ
BaCl2
BaCl2
�������Լ�I�ٶ�Ӧ��ʵ�����������
����
�����з�����Ӧ�����ӷ���ʽΪBa2++SO42-=BaSO4������Ba2++CO32-=BaCO3��
Ba2++SO42-=BaSO4������Ba2++CO32-=BaCO3��
����3���Լ���Ļ�ѧʽΪ
K2CO3
K2CO3
�����м����Լ����Ŀ������ȥ�����Ba2+
��ȥ�����Ba2+
����4���Լ����������
����
����
�����з�����Ӧ�����ӷ���ʽΪ2H++CO32-=H2O+CO2��
2H++CO32-=H2O+CO2��
����5��ijͬѧ��ȡ�ᴿ�IJ�Ʒ0.745g���ܽ������100mL����ƿ�У�ÿ��ȡ25.00mL��Һ����0.1000mol?L-1������������Һ��Ӧ�����η�Ӧ��������������Һ��ƽ�����Ϊ23.50mL���ò�Ʒ�Ĵ���Ϊ
94%
94%
����������1����������Һ�м��������ữ���ټ���BaCl2��Һ������BaSO4��ɫ������������֤����SO42-��
��2�����Ȼ�����Һ����ͬʱ��ȥ��������Ӻ�̼������ӣ��������ӷ���ʽ����д������д��
��3��Ϊ��ȥ�����ı�����ѡ��K2CO3���Լ��������ܳ�ȥ������ͬʱ�������Ȼ��أ�
��4������������ȥ������̼������ӣ��������ӷ���ʽ����д������д��ע��������ʺ�����д��ѧʽ��
��5���ȸ��������������ʵ�������25mL�Ȼ�����Һ�к��е��Ȼ��ص����ʵ������ټ���100mL�Ȼ�����Һ�к��е��Ȼ��ص����ʵ������Ӷ����������������������������ʽ�����������������ɣ�
��2�����Ȼ�����Һ����ͬʱ��ȥ��������Ӻ�̼������ӣ��������ӷ���ʽ����д������д��
��3��Ϊ��ȥ�����ı�����ѡ��K2CO3���Լ��������ܳ�ȥ������ͬʱ�������Ȼ��أ�
��4������������ȥ������̼������ӣ��������ӷ���ʽ����д������д��ע��������ʺ�����д��ѧʽ��
��5���ȸ��������������ʵ�������25mL�Ȼ�����Һ�к��е��Ȼ��ص����ʵ������ټ���100mL�Ȼ�����Һ�к��е��Ȼ��ص����ʵ������Ӷ����������������������������ʽ�����������������ɣ�
����⣺��1����������Һ�м��������ữ���ټ���BaCl2��Һ������BaSO4��ɫ������������֤����SO42-����֮���ޣ��ʴ�Ϊ��ȡ������Һ���Թ��У������м��������ữ��BaCl2��Һ�����а�ɫ�������ɣ�����SO42-����֮���ޣ�
��2��Ҫ�������������������̼�����Ӧ����������Ȼ�����Һ��̼�������������ɲ�����ˮ�ı��Σ�ͬʱ�����Ȼ��أ����ӷ���ʽΪ��SO42-+Ba2+=BaSO4����CO32-+Ba2+=BaCO3����
�ʴ�Ϊ��BaCl2�����ˣ�Ba2++SO42-=BaSO4����Ba2++CO32-=BaCO3����
��3��Ҫ��������ı����ӣ�Ҫ����̼��أ�̼��غ��Ȼ�����Ӧ����̼�ᱵ����ͬʱ�����Ȼ��أ����ӷ���ʽΪ
CO32-+Ba2+=BaCO3�����ʴ�Ϊ��K2CO3����ȥ�����Ba2+��
��4��Ҫ���������̼�����Ҫ�μ����������ᣬ̼������Ӻ����ᷴӦ���ɶ�����̼��ˮ�����ӷ���ʽΪCO32-+2H+=CO2��+H2O���ʴ�Ϊ�����2H++CO32-=H2O+CO2����
��5����25mL�Ȼ�����Һ���Ȼ��ص����ʵ���Ϊnmol��
KCl+AgNO3=AgCl+KNO 3
1mol 1mol
nmol 0.1000mol?L-1 ��0.02350L
n=0.002350mol
100mL��Һ�к����Ȼ��ص����ʵ���=0.002350mol��4=0.0094mol
100mL��Һ�к����Ȼ��ص�����=0.0094mol��74.5g/mol=0.7003g
��������=
��100%=94%���ʴ�Ϊ��94%��
��2��Ҫ�������������������̼�����Ӧ����������Ȼ�����Һ��̼�������������ɲ�����ˮ�ı��Σ�ͬʱ�����Ȼ��أ����ӷ���ʽΪ��SO42-+Ba2+=BaSO4����CO32-+Ba2+=BaCO3����
�ʴ�Ϊ��BaCl2�����ˣ�Ba2++SO42-=BaSO4����Ba2++CO32-=BaCO3����
��3��Ҫ��������ı����ӣ�Ҫ����̼��أ�̼��غ��Ȼ�����Ӧ����̼�ᱵ����ͬʱ�����Ȼ��أ����ӷ���ʽΪ
CO32-+Ba2+=BaCO3�����ʴ�Ϊ��K2CO3����ȥ�����Ba2+��
��4��Ҫ���������̼�����Ҫ�μ����������ᣬ̼������Ӻ����ᷴӦ���ɶ�����̼��ˮ�����ӷ���ʽΪCO32-+2H+=CO2��+H2O���ʴ�Ϊ�����2H++CO32-=H2O+CO2����
��5����25mL�Ȼ�����Һ���Ȼ��ص����ʵ���Ϊnmol��
KCl+AgNO3=AgCl+KNO 3
1mol 1mol
nmol 0.1000mol?L-1 ��0.02350L
n=0.002350mol
100mL��Һ�к����Ȼ��ص����ʵ���=0.002350mol��4=0.0094mol
100mL��Һ�к����Ȼ��ص�����=0.0094mol��74.5g/mol=0.7003g
��������=
| 0.7003g |
| 0.745g |
���������⿼���˳����Լ���ѡȡ�����ӷ���ʽ����д��֪ʶ�㣬�ѶȲ���ע������������ʱ��ץס�����ʵı���������������Լ�ֻ�����ʷ�Ӧ����Ӧ���������µ����ʣ�����ȷ����Ĺؼ���

��ϰ��ϵ�д�
 ͨ��ѧ��Ĭд����ϵ�д�
ͨ��ѧ��Ĭд����ϵ�д�
�����Ŀ



