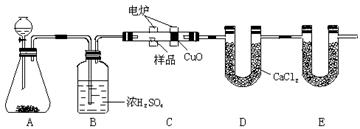
��Ŀ����
����ʵ������У���ȷ����
| A��ϡ��ŨH2SO4ʱ��������ˮ�������ڱڻ�������ŨH2SO�У�����ʱ���� |
| B���ⶨ��ҺpHʱ���ýྻ�IJ�����պȡ����Һ����pH��ֽ�ϣ��۲���ֽ����ɫ�仯���������ɫ����Ƚ� |
| C������NaOH����ʱ����NaOH������ڵ�����ֽ�������� |
| D��������NaCl��Һ�õ�NaCl�����ʵ���У�������������е�ˮ��ȫ�����ɺ���ܳ�ȥ�ƾ��� |
B
���������ϡ��ŨH2SO4ʱ�����������������ڱڻ�������ˮ�У�����ʱ���裬A����ȷ�����������׳��⣬�Ҽ��鸯ʴ�ԣ�Ӧ�÷����ձ��г�����C����ȷ��D����ȷ������ʱ������ִ����ľ����Ǿ�Ӧ��ֹͣ���ȣ���ѡB��
�����������Ǹ߿��еij������ͣ������е��Ѷȵ����⣬���������ǿ������������Ҫ���Գ���������ѡ�á�ʵ���������Ϊ���ģ�ͨ����ʲô��Ϊʲô���������ص㿼��ʵ����������Ĺ淶�Ժ�ȷ���������֪ʶ���ʵ�������������

��ϰ��ϵ�д�
�����Ŀ