��Ŀ����
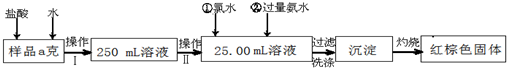
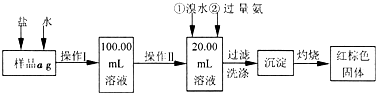
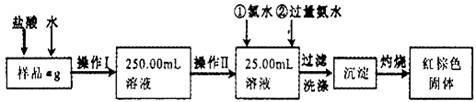
ij�Ȼ�����Ʒ��������FeCl2���ʡ���Ҫ�ⶨ������Ԫ�ص�����������ʵ�鰴���²�����У�
������������̣��ش��������⣺
��1������I���õ��IJ����������ձ�������������Ͳ�⣬��������____________ ������ѡ�������ƣ�������II�����õ���������____________�����ţ���
����
| A��50mL�ձ� | B��50mL��Ͳ ���� | C��25mL��ʽ�ζ��� | D��25mL��ʽ�ζ��� |
��3����������Ƿ��Ѿ�ϴ�Ӹɾ��IJ�����________________________��
��4������������ȣ������ڸ���������ȴ�����£�����ƽ����������Ϊb1g���ٴμ��Ȳ���ȴ�����³���������Ϊb2g����b1��b2=0.3g�����������Ӧ���еIJ�����____________��
��5����������������W1g������������Ⱥ������������W2g������Ʒ����Ԫ�ص�����������____________��
��6����ͬѧ��Ϊ����������������������ˮ���������費�䣬�ԿɴﵽĿ�ġ�����������________________________�����û�ѧ����ʽ��ʾ��
��1��250mL����ƿ����ͷ�ιܣ�D
��2��NH+4��Cl-��OH-
��3��ȡ�������һ��ϴ��Һ���Թ��У��μ������ữ��AgNO3��Һ�����������ɣ���֤��ԭ����ϴ�Ӹɾ����������Ĵ𰸾��ɵ÷֣�
��4���ٴμ��ȣ������ڸ���������ȴ��������ֱ���������������0.1gΪֹ��
��5��
��6��4Fe��OH��2��2H2O��O2=4Fe��OH��3
����

��ϰ��ϵ�д�
 ǧ�������������ĩ�����Ծ�����ϵ�д�
ǧ�������������ĩ�����Ծ�����ϵ�д�
�����Ŀ