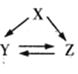��Ŀ����
����Ŀ����A��B��C��D���ֳ����Ľ������ʣ���AԪ�ص���������ʱ�ᷢ�������Ļ�ɫ���棻BΪ�Ϻ�ɫ���壬��ʴʱ��Ϊ��ɫ��C�ڿ����м����ۻ��������䣻D��������ȼ�գ��������䡣����������Ϣ�ش��������⣺
(1)д����Ӧ��ѧʽ��A________��B________��C________��D________��
(2)B��ʴʱ���ɵ���ɫ���ʵ���Ҫ�ɷ���________��D��������ȼ�����ɵ�������Ϊ________��
(3)д�����з�Ӧ����ʽ��
��A��̼���������ȷֽ�Ļ�ѧ����ʽ______________________________________��
��C�������ռ���Һ��Ӧ�����ӷ���ʽ______________________________________��
��B��D����������Һ��Ӧ�����ӷ���ʽ____________________________________��
���𰸡�Na Cu Al Fe Cu2(OH)2CO3 Fe3O4 2NaHCO3![]() Na2CO3+CO2��+H2O 2Al+2OH-+2H2O=2AlO2-+3H2�� Cu+2Fe3+=Cu2++2Fe2+
Na2CO3+CO2��+H2O 2Al+2OH-+2H2O=2AlO2-+3H2�� Cu+2Fe3+=Cu2++2Fe2+
��������
��A��B��C��D���ֳ����Ľ������ʣ���AԪ�ص���������ʱ�ᷢ�������Ļ�ɫ���棬��AΪNa��BΪ�Ϻ�ɫ���壬��ʴʱ��Ϊ��ɫ����BΪCu��C�ڿ����м����ۻ��������䣬CΪAl��D��������ȼ�գ��������䣬��DΪFe��Ȼ��������ʵ����ʼ��仯�������
(1)��������������֪��A��Na��B��Cu��C��Al��D��Fe��
(2)Cu�������ڿ����б�Ϊ��ɫ����Ϊ��ʽ̼��ͭ����ѧʽΪ��Cu2(OH)2CO3��
Fe��������ȼ�գ��������䣬���ɵ�������ΪFe3O4��
(3)��A��̼������NaHCO3���ȶ������ȷֽ⣬����̼���ơ�ˮ��������̼���ֽⷴӦ�Ļ�ѧ����ʽΪ��2NaHCO3![]() Na2CO3+CO2��+H2O��
Na2CO3+CO2��+H2O��
��C��Al��Al�������ռ���Һ��Ӧ����NaAlO2��H2����Ӧ�����ӷ���ʽΪ��2Al+2OH-+2H2O=2AlO2-+3H2����
��Cu���ʾ��л�ԭ�ԣ�Fe3+���������ԣ���������Һ�з���������ԭ��Ӧ������Cu2+��Fe2+����Ӧ�����ӷ���ʽΪ��Cu+2Fe3+=Cu2++2Fe2+��

 ��Ӣ���㿨ϵ�д�
��Ӣ���㿨ϵ�д� Ӧ����㲦ϵ�д�
Ӧ����㲦ϵ�д� ״Ԫ����ϵ�д�
״Ԫ����ϵ�д� ͬ������ϵ�д�
ͬ������ϵ�д�����Ŀ���±��������ʣ��������ǵ���Һ��ͨ��һ����Ӧ��ʵ����ͼ��ʾ��ת������
ѡ�� | X | Y | Z |
|
A | Si | Na2SiO3 | H2SiO3 | |
B | S | H2S | SO2 | |
C | Al2O3 | NaAlO2 | Al2(SO4)3 | |
D | Mg(OH)2 | MgCO3 | MgCl2 |
A.AB.BC.CD.D