��Ŀ����
 ��ͼװ�ÿ����ڶ����ʵ�飮ͼ�мг̶ֹ�װ������ȥ�����п̶ȣ��������ã�
��ͼװ�ÿ����ڶ����ʵ�飮ͼ�мг̶ֹ�װ������ȥ�����п̶ȣ��������ã��� 1 ��װ�����п̶ȵļܿ�����
�� 2 ��ijʵ��С����þ�ۡ����ᡢ�������ʵ����֤������ͬ��ͬѹ�£�����������������ʵ�����ͬʱ����þ�۷�Ӧ���������������ͬ����Ӧ���ʲ�ͬ��װ����ͼ��ʾ��
�й�ʵ�����ݼ�¼���±���
| ����Һ | ����Һ | �������/mL | ��Ӧʱ�� | |
| ��ʵ��A�� | ��ʵ��B�� | ��25�桢101 kPa�� | ʵ��A | ʵ��B |
| CH3COOH0.1 mol/L40.00mL | HCl��Һ 0.1 mol/L 40.00mL |
5 | t��a1��=155 s | t��b1��=7 s |
| 10 | t��a2��=310 s | t��b2��=16 s | ||
| 15 | t��a3��=465 s | t��b3��=30 s | ||
| 20 | t��a4��=665 s | t��b4��=54 s | ||
| �� | �� | �� | ||
��ÿ��ʵ��������Ҫ�õ�����ƽ���ܳ�1mg�� ��ȡþ��
����ȴ��25����ڶ�ȡ�������ʱ������Ӧ��β�����
�۷���ʵ�����ݣ�t��a1��ԶԶ����t��b1����ԭ����
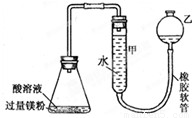
��3����ͼʾװ�ã�ijͬѧ����˲ⶨ��п��Ƥ�Ʋ��ȵ�ʵ�鷽�������������ΪS cm2������Ϊmg�Ķ�п��Ƥ��6mol?L-1 NaOH��Һ��Ӧ���ش��������⣺����֪п���ܶ�Ϊ �� g/cm3��
��д��Zn�Ʋ���NaOH��Һ��Ӧ�����ӷ���ʽ
��Ϊ��߲ⶨ��ȷ�ԣ��轫��ƿ�ϵĵ���������Ϊ˫����������һ�ײ���
ʵ��ʱ������ƿ�м����п��Ƥ��Ʒ������˫�������ټ���NaOH��Һ��
����֪ʵ��ǰ�����Һ�������ΪV mL��ʵ������������Ħ�����ΪVm mol?L-1�������п��Ƥ��п�Ʋ���Ϊ
| 65V��10-3 |
| 2��SVm |
| 65V��10-3 |
| 2��SVm |
��2���ٸ�����ͽ���֮��Ĺ�ϵʽ���㣻
����ȴ��25����ڶ�ȡ�������ʱ��Ҫʹ����������Һ����ƽʱ�ٶ�����
�����ʵ���Ũ�ȴ�СӰ�컯ѧ��Ӧ���ʽ�����Ӱ�컯ѧ��Ӧ��ʱ�䣻
��3����п�����ʺ������������ƣ����������������������Ƶķ�Ӧ����ʽд��������п������������Һ�ķ�Ӧ����ʽ��
���÷�Һ©������ƿ�ڼ�������������Һ�����ò������������ѹǿʱ����������Һ���£�
�۸���������п֮��Ĺ�ϵʽ���㣮
�ʴ�Ϊ����ʽ�ζ��ܣ�������ע��������ˮ��ʹ����Һ����ڼ���Һ�棬��ֹ�۲죬��Һ���ֲ��䣬�����������ã�
��2���ٸ�����ͽ���֮��Ĺ�ϵʽ��þ������=
| 0.1mol/L��0.04L |
| 2 |
�ʴ�Ϊ��0.048��
����ȴ��25����ڶ�ȡ�������ʱ��Ҫʹ����������Һ����ƽʱ�ٶ��������Һ�治��ƽ����Һ�����ѹǿ���Բ��������Ӱ�죬
�ʴ�Ϊ�������ƶ��ҹܣ�ʹ����Һ����ҹ���Һ����ƽ���ٶ�����
�۴��������ᣬ����̶Ⱥ�С��������ǿ�ᣬ���Կ�ʼ�δ�����Һ�е�[H+]ԶС����ͬŨ�ȵ������е�[H+]������a1�ķ�Ӧ����С��b1����t��a1��ԶԶ����t��b1����
�ʴ�Ϊ����ʼ�δ�����Һ�е�c��H+��ԶС����ͬŨ�ȵ������е�c��H+����
��3����п�����ʺ������������ƣ����������������������Ƶķ�Ӧ����ʽ֪��������п������������Һ��Ӧ�����ӷ���ʽΪ��Zn+2OH-+2H2O=Zn��OH��42-+H2����
�ʴ�Ϊ��Zn+2OH-+2H2O=Zn��OH��42-+H2����
���÷�Һ©������ƿ�ڼ�������������Һ�����ò������������ѹǿʱ����������Һ���£�������һ�ײ����Һ©�����ʴ�Ϊ����Һ©����
����пƬ�ĺ��Ϊhcm������ת�Ƶ�����ȵã�п������֮��Ĺ�ϵʽΪ��
| ��cm3?2scm2��hcm |
| 65g/mol |
| 10-3VL |
| VmL/mol |
| 65V��10-3 |
| 2��SVm |
�ʴ�Ϊ��
| 65V��10-3 |
| 2��SVm |

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����ͼװ�ÿ����ڶ����ʵ�顣ͼ�мг̶ֹ�װ������ȥ�����п̶ȣ��������á�

( 1 )װ�����п̶ȵļܿ�����______________���� (����������)����ͼ���Ӻ�װ�ú��װ�������Եķ�����______________________________
( 2 )ijʵ��С����þ�ۡ����ᡢ�������ʵ����֤������ͬ��ͬѹ�£�����������������ʵ�����ͬʱ����þ�۷�Ӧ���������������ͬ����Ӧ���ʲ�ͬ��װ������ͼ��ʾ��
�й�ʵ�����ݼ�¼���±���
|
����Һ |
����Һ |
���������mL |
��Ӧʱ�� |
|
|
(ʵ��A) |
(ʵ��B) |
(25�桢101 kPa) |
ʵ��A |
ʵ��B |
|
CH3COOH 0.1 mol��L 40.00mL |
HCl��Һ 0.1 mol��L 40.00mL |
5 |
t(a1)=155 s |
t(b1)=7 s |
|
10 |
t(a2)=310 s |
t(b2)=16 s |
||
|
15 |
t(a3)=465 s |
t(b3)=30 s |
||
|
20 |
t(a4)=665 s |
t(b4)=54 s |
||
|
���� |
���� |
���� |
��ش��������⣺
��ÿ��ʵ��������Ҫ�õ�����ƽ(�ܳ�1 mg) ��ȡþ��___________________g��
����ȴ��25����ڶ�ȡ�������ʱ������Ӧ��β�����__________________________��
�۷���ʵ�����ݣ�t(a1)ԶԶ����t(b1)��ԭ����__________________________��
��3����ͼʾװ��,ijͬѧ����˲ⶨ��п��Ƥ�Ʋ��ȵ�ʵ�鷽�������������ΪS cm2������Ϊm g�Ķ�п��Ƥ��6mol��L��1 NaOH��Һ��Ӧ���ش��������⣺����֪п���ܶ�Ϊ �� g/cm3��
�� д��Zn�Ʋ���NaOH��Һ��Ӧ�����ӷ���ʽ________________________________________
��Ϊ��߲ⶨ��ȷ�ԣ��轫��ƿ�ϵĵ���������Ϊ˫����������һ�ײ���______������������)
ʵ��ʱ������ƿ�м����п��Ƥ��Ʒ������˫�������ټ���NaOH��Һ��
�� ��֪ʵ��ǰ�����Һ�������ΪV mL��ʵ������������Ħ�����ΪVm mol��L��1�������п��Ƥ��п�Ʋ���Ϊ_________________________cm����д����ѧ����ʽ��
 ��ͼװ�ÿ����ڶ����ʵ�飮ͼ�мг̶ֹ�װ������ȥ�����п̶ȣ��������ã�
��ͼװ�ÿ����ڶ����ʵ�飮ͼ�мг̶ֹ�װ������ȥ�����п̶ȣ��������ã�
�� 1 ��װ�����п̶ȵļܿ�����________���� �����������ƣ�����ͼ���Ӻ�װ�ú��װ�������Եķ�����________
�� 2 ��ijʵ��С����þ�ۡ����ᡢ�������ʵ����֤������ͬ��ͬѹ�£�����������������ʵ�����ͬʱ����þ�۷�Ӧ���������������ͬ����Ӧ���ʲ�ͬ��װ����ͼ��ʾ��
�й�ʵ�����ݼ�¼���±���
| ����Һ | ����Һ | �������/mL | ��Ӧʱ�� | |
| ��ʵ��A�� | ��ʵ��B�� | ��25�桢101 kPa�� | ʵ��A | ʵ��B |
| CH3COOH0.1 mol/L40.00mL | HCl��Һ 0.1 mol/L 40.00mL | 5 | t��a1��=155 s | t��b1��=7 s |
| 10 | t��a2��=310 s | t��b2��=16 s | ||
| 15 | t��a3��=465 s | t��b3��=30 s | ||
| 20 | t��a4��=665 s | t��b4��=54 s | ||
| �� | �� | �� | ||
��ÿ��ʵ��������Ҫ�õ�����ƽ���ܳ�1mg�� ��ȡþ��________g��
����ȴ��25����ڶ�ȡ�������ʱ������Ӧ��β�����________��
�۷���ʵ�����ݣ�t��a1��ԶԶ����t��b1����ԭ����________��
��3����ͼʾװ�ã�ijͬѧ����˲ⶨ��п��Ƥ�Ʋ��ȵ�ʵ�鷽�������������ΪS cm2������Ϊmg�Ķ�п��Ƥ��6mol?L-1 NaOH��Һ��Ӧ���ش��������⣺����֪п���ܶ�Ϊ �ѡ�g/cm3��
��д��Zn�Ʋ���NaOH��Һ��Ӧ�����ӷ���ʽ________
��Ϊ��߲ⶨ��ȷ�ԣ��轫��ƿ�ϵĵ���������Ϊ˫����������һ�ײ���________�����������ƣ�
ʵ��ʱ������ƿ�м����п��Ƥ��Ʒ������˫�������ټ���NaOH��Һ��
����֪ʵ��ǰ�����Һ�������ΪV mL��ʵ������������Ħ�����ΪVm mol?L-1�������п��Ƥ��п�Ʋ���Ϊ________cm����д����ѧ����ʽ��
�� 1 ��װ�����п̶ȵļܿ����� ���� �����������ƣ�����ͼ���Ӻ�װ�ú��װ�������Եķ�����
�� 2 ��ijʵ��С����þ�ۡ����ᡢ�������ʵ����֤������ͬ��ͬѹ�£�����������������ʵ�����ͬʱ����þ�۷�Ӧ���������������ͬ����Ӧ���ʲ�ͬ��װ����ͼ��ʾ��
�й�ʵ�����ݼ�¼���±���
| ����Һ | ����Һ | �������/mL | ��Ӧʱ�� | |
| ��ʵ��A�� | ��ʵ��B�� | ��25�桢101 kPa�� | ʵ��A | ʵ��B |
| CH3COOH0.1 mol/L40.00mL | HCl��Һ 0.1 mol/L 40.00mL | 5 | t��a1��=155 s | t��b1��=7 s |
| 10 | t��a2��=310 s | t��b2��=16 s | ||
| 15 | t��a3��=465 s | t��b3��=30 s | ||
| 20 | t��a4��=665 s | t��b4��=54 s | ||
| �� | �� | �� | ||
��ÿ��ʵ��������Ҫ�õ�����ƽ���ܳ�1mg�� ��ȡþ�� g��
����ȴ��25����ڶ�ȡ�������ʱ������Ӧ��β����� ��
�۷���ʵ�����ݣ�t��a1��ԶԶ����t��b1����ԭ���� ��
��3����ͼʾװ�ã�ijͬѧ����˲ⶨ��п��Ƥ�Ʋ��ȵ�ʵ�鷽�������������ΪS cm2������Ϊmg�Ķ�п��Ƥ��6mol?L-1 NaOH��Һ��Ӧ���ش��������⣺����֪п���ܶ�Ϊ �� g/cm3��
��д��Zn�Ʋ���NaOH��Һ��Ӧ�����ӷ���ʽ
��Ϊ��߲ⶨ��ȷ�ԣ��轫��ƿ�ϵĵ���������Ϊ˫����������һ�ײ��� �����������ƣ�
ʵ��ʱ������ƿ�м����п��Ƥ��Ʒ������˫�������ټ���NaOH��Һ��
����֪ʵ��ǰ�����Һ�������ΪV mL�����п��Ƥ��п�Ʋ���Ϊ cm����д����ѧ����ʽ��

 s
s ________��
________��