��Ŀ����
����Ŀ���о������к�����(��Ҫ��SO2��H2S)��ת��������Ҫ���塣
��1����ʪ�����£�д��������SO2ת��ΪHSO3-�ķ���ʽ��____________��____________��
��2�������е�����ɽ�������H2S��������Ӧ������SO42-��������Ӧ�������仯ʾ��ͼ���£�

1mol H2S(g)ȫ��������SO42-(aq)���Ȼ�ѧ����ʽΪ_____________________��
��3���������������ӽ���Ĥȼ�ϵ�ؿ������ô�������SO2������������װ��ʾ��ͼ���£�

�����ӵ���������Ϊ_________________������A��B����B��A������
�ڸ����ĵ缫��ӦʽΪ__________________________��
��4��ȼú��������������Ǽ��ٴ����к�������Ⱦ�Ĺؼ���SO2�����ѳ���һ�ֹ�ҵ�������£�

�� �ô�����Һ����SO2����ת��ΪHSO3-����Ӧ�����ӷ���ʽ��________________��
�� ��ʯ�������������������Ż����ճأ����п���������SO2�����ʵĻ�ѧʽ��_____________��
���𰸡���1��SO2+H2O![]() H2SO3��H2SO3
H2SO3��H2SO3![]() H++HSO3-
H++HSO3-
��2��H2S(g)+2O2(g)=SO42-(aq)+2H+(aq)��H=��806.39kJ��mol��1
��3������A��B(1��)��SO2�C2e-+2H2O==SO42-+4H+
��4����H2O+2SO2+CO32-��2HSO3-+CO2�� ��NaOH
��������
�����������1����ʪ��������������SO2ת��ΪHSO3-�ķ���ʽΪSO2+H2O![]() H2SO3��H2SO3
H2SO3��H2SO3![]() H++HSO3-��
H++HSO3-��
��2������ͼ���У���H2S(g)+![]() O2(g)��S(s)+H2O(g) ��H��-221.19kJ/mol����S(s)+
O2(g)��S(s)+H2O(g) ��H��-221.19kJ/mol����S(s)+![]() O2(g)�� SO42-(aq) +2H+(aq) ��H��-585.20kJ/mol������+�����ã�H2S(g) + 2O2(g)��SO42-(aq) + 2H+(aq) ��H��-221.19kJ/mol+��-585.20kJ/mol������806.39 kJ/mol��
O2(g)�� SO42-(aq) +2H+(aq) ��H��-585.20kJ/mol������+�����ã�H2S(g) + 2O2(g)��SO42-(aq) + 2H+(aq) ��H��-221.19kJ/mol+��-585.20kJ/mol������806.39 kJ/mol��
��3��������ͼʾ��AΪȼ�ϵ�صĸ�����BΪȼ�ϵ�ص���������ԭ��ص��Һ�У�������ɸ����������ƶ�������A��B������������������Ӧ��������������Ϊ������������缫��ӦʽΪSO2�C2e-+2H2O��SO42-+4H+��
��4����̼������Һ����SO2�������������ƺͶ�����̼��������Ӧ�����ӷ���ʽΪH2O+2SO2 +CO32-��2HSO3- +CO2�������������������ճ�����̼���Ʒ�Ӧ����̼��Ƴ������������ƣ������������ƿ�������SO2��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ����1����ѧƽ�ⳣ��K��ʾ���淴Ӧ�Ľ��г̶ȣ�KֵԽ��ʾ�� Kֵ��С���¶ȵĹ�ϵ�ǣ��¶����ߣ�Kֵ ������һ������һ����С�����������Ҳ���ܼ�С����
��2����һ���Ϊ10L�������У�ͨ��һ������CO��H2O����850��ʱ�������·�Ӧ��CO��g�� +H2O��g�� ![]() CO2��g�� +H2 ��g�� ��H<0��CO��H2OŨ�ȱ仯����ͼ����0��4min��ƽ����Ӧ����v��CO����mol��L-1��min-1��t��ʱ����Ũ����mol��L-1���ı仯��
CO2��g�� +H2 ��g�� ��H<0��CO��H2OŨ�ȱ仯����ͼ����0��4min��ƽ����Ӧ����v��CO����mol��L-1��min-1��t��ʱ����Ũ����mol��L-1���ı仯��
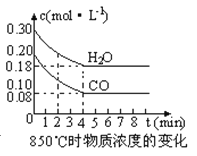
ʱ����min�� | CO | H2O | CO2 | H2 |
0 | 0.200 | 0.300 | 0 | 0 |
2 | 0.138 | 0.238 | 0.062 | 0.062 |
3 | c1 | c2 | c3 | c3 |
4 | c1 | c2 | c3 | c3 |
5 | 0.116 | 0.216 | 0.084 | |
6 | 0.096 | 0.266 | 0.104 |
��3��t��������850����ʱ������ͬ�����з���������Ӧ�������ڸ����ʵ�Ũ�ȱ仯���ϱ���
�ٱ���3min��4min֮�䷴Ӧ���� ״̬��c 1��ֵ 0.08 mol��L-1 ������ڡ�С�ڻ��������
�ڷ�Ӧ��4min��5min�䣬ƽ�����淽���ƶ������ܵ�ԭ���� ����ѡ��������5min��6min֮����ֵ�����仯�����ܵ�ԭ���� ����ѡ����
a������ˮ���� b�������¶� c��ʹ�ô��� d����������Ũ��