ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩ÷ΊΗθΥαΦΊ”ΟΆΨΦΪΙψΓΘΗθΧζΩσΒΡ÷ς“Σ≥…Ζ÷ΈΣFeOΓΛCr2O3Θ§Κ§”–SiO2ΓΔAl2O3Β»‘”÷ ΓΘ“‘ΗθΧζΩσΈΣ‘≠Νœ÷Τ±ΗK2Cr2O7ΨßΧεΒΡΙΐ≥Χ»γΆΦΥυ ΨΘΚ
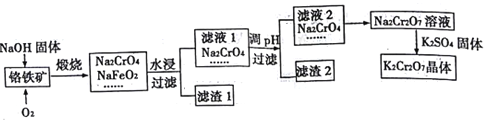
“―÷ΣΘΚΔΌNaFeO2”ωΥ°«ΩΝ“Υ°Ϋβ ΔΎCr2072-+H2O=2CrO42-+2H+
«κΜΊ¥πΘΚ
Θ®1Θ©ΕΆ…’ΗθΧζΩσΒΡ÷ς“ΣΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ Ϋ «______________ΓΘ
Θ®2Θ©¬Υ“ΚIΒΡ≥…Ζ÷≥ΐNaOHΓΔNa2CrO4ΆβΘ§ΜΙΚ§”–____________Θ®ΧνΜ·―ß ΫΘ©ΘΜ¬Υ‘ϋI÷–”–ΚλΚ÷…ΪΈο÷ Θ§…ζ≥…ΗΟΈο÷ ΒΡΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ Ϋ «______________ΓΘ
Θ®3Θ©”…¬Υ“Κ2ΉΣΜ·ΈΣNa2Cr207»ή“Κ”ΠΦ”»κΒΡ ‘ΦΝ «________ΓΘ
Θ®4Θ©œρNa2Cr207»ή“ΚΦ”»κK2SO4ΙΧΧεΘ§ΖΔ…ζΗ¥Ζ÷ΫβΖ¥”ΠΕχ÷ΤΒΟK2Cr207ΓΘ…ζ≥…K2Cr207ΨßΧεΒΡΙΐ≥Χ «ΘΚΦ”»»≈®ΥθΓΔά以ΫαΨßΓΔΙΐ¬ΥΓΔœ¥Β”ΓΔΗ…‘οΓΘ ‘Φρ ω¥ΥΖ®ΡήΙΜ÷ΤΒΟK2Cr207ΨßΧεΒΡ‘≠“ρΘΚ______ΘΜœ¥Β” ±”Π―Γ”Ο________Θ®―ΓΧνΉ÷ΡΗΘ©ΘΜ
A.’τΝσΥ° B.œΓΝρΥα C.±ΞΚΆK2SO4»ή“Κ
»γΚΈΦλ―ιΨßΧε“―Ψ≠œ¥Β”Η…ΨΜΘΚ______________ΓΘ
ΓΨ¥πΑΗΓΩ 4FeOΓΛCr2O3+7O2+20NaOH![]() 8Na2CrO4+4NaFeO2+10H2O NaAlO2ΓΔNaSiO3 FeO2-+2H2O=OH-+Fe(OH)3Γΐ Θ®œΓΘ©ΝρΥα Ά§Έ¬œ¬K2Cr2O7ΨßΧεΒΡ»ήΫβΕ»–Γ”ΎNa2Cr2O7 A »ΓΉνΚσ“Μ¥Έœ¥Β”“Κ…Ό–μ”Ύ ‘Ιή÷–Θ§Φ”BaCl2»ή“ΚΘ§»γΈόΑΉ…Ϊ≥ΝΒμΘ§‘ρ“―œ¥Β””ΎΨΜ
8Na2CrO4+4NaFeO2+10H2O NaAlO2ΓΔNaSiO3 FeO2-+2H2O=OH-+Fe(OH)3Γΐ Θ®œΓΘ©ΝρΥα Ά§Έ¬œ¬K2Cr2O7ΨßΧεΒΡ»ήΫβΕ»–Γ”ΎNa2Cr2O7 A »ΓΉνΚσ“Μ¥Έœ¥Β”“Κ…Ό–μ”Ύ ‘Ιή÷–Θ§Φ”BaCl2»ή“ΚΘ§»γΈόΑΉ…Ϊ≥ΝΒμΘ§‘ρ“―œ¥Β””ΎΨΜ
ΓΨΫβΈωΓΩ(1)Ά®Ιΐ“‘…œΖ÷Έω÷ΣΘ§ΓΑ±Κ…’Γ±ΚσΒΡΙΧΧε≤ζΈο≥…Ζ÷≥ΐNa2CrO4ΓΔNaFeO2ΆβΘ§ΜΙ”–NaAlO2ΓΔNa2SiO3ΘΜ±Κ…’Ιΐ≥Χ÷–ΖΔ…ζΒΡ÷ς“ΣΖ¥”ΠΈΣ4FeOΓΛCr2O3+7O2+20NaOH![]() 8Na2CrO4+4NaFeO2+10H2OΘΜ
8Na2CrO4+4NaFeO2+10H2OΘΜ
(2)«β―θΜ·ΡΤΚΆ―θΜ·¬ΝΖ¥”Π…ζ≥…ΤΪ¬ΝΥαΡΤΘ§ΚΆΕΰ―θΜ·ΙηΖ¥”Π…ζ≥…ΙηΥαΡΤΘ§¬Υ“ΚIΒΡ≥…Ζ÷≥ΐNaOHΓΔNa2CrO4ΆβΘ§ΜΙΚ§”–NaAlO2ΓΔNaSiO3 ΘΜ¬Υ‘ϋI÷–”–ΚλΚ÷…ΪΈο÷ ΈΣFe(OH)3ΓΐΘ§…ζ≥…ΗΟΈο÷ ΒΡΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΈΣFeO2-+2H2O=OH-+Fe(OH)3ΓΐΘΜ
(3)”…¬Υ“Κ2÷–¥φ‘Ύ2H++2CrO42-![]() Cr2O72-+H2OΘ§Φ”»κœΓΝρΥαΘ§Ω…¥ΌΫχΤΫΚβ’ΐœρ“ΤΕ·Θ§ ΙNa2CrO4ΉΣΜ·ΈΣNa2Cr2O7ΘΜ
Cr2O72-+H2OΘ§Φ”»κœΓΝρΥαΘ§Ω…¥ΌΫχΤΫΚβ’ΐœρ“ΤΕ·Θ§ ΙNa2CrO4ΉΣΜ·ΈΣNa2Cr2O7ΘΜ
(4)“ρΆ§Έ¬œ¬K2Cr2O7ΨßΧεΒΡ»ήΫβΕ»–Γ”ΎNa2Cr2O7 Θ§œρNa2Cr2O7»ή“ΚΦ”»κK2SO4ΙΧΧεΘ§»ΜΚσΫΪ»ή“ΚΦ”»»≈®ΥθΓΔά以ΫαΨßΓΔΙΐ¬ΥΓΔœ¥Β”ΓΔΗ…‘οΘ§Φ¥Ω…ΜώΒΟK2Cr2O7ΨßΧεΘΜœ¥Β” ±ΈΣ±ήΟβ“ΐ»κ–¬ΒΡ‘”÷ Θ§―Γ‘ώΥ°œ¥Θ§Φ¥¥πΑΗΈΣAΘΜΨßΧε±μΟφΗΫΉ≈“ΚάοΚ§”–SO42-Θ§»γ»ΓΉνΚσ“Μ¥Έœ¥Β”“Κ…Ό–μ”Ύ ‘Ιή÷–Θ§Φ”BaCl2»ή“ΚΘ§»γΈόΑΉ…Ϊ≥ΝΒμΘ§‘ρ“―œ¥Β””ΎΨΜΓΘ

 ΒΎ1ΨμΒΞ‘Σ‘¬ΩΦΤΎ÷–ΤΎΡ©œΒΝ–¥πΑΗ
ΒΎ1ΨμΒΞ‘Σ‘¬ΩΦΤΎ÷–ΤΎΡ©œΒΝ–¥πΑΗΓΨΧβΡΩΓΩœ¬Ν–ΗςΉιΈο÷ ΡήΑ¥’’»γΆΦΙΊœΒΆΦΘ®ΓΑΓζΓ±±μ ΨΖ¥”Π“Μ≤ΫΆξ≥…Θ©œύΜΞΉΣΜ·ΒΡ «

A | B | C | D | |
X | NaOH | Cu | Fe2O3 | Ca(OH)2 |
Y | NaNO3 | CuO | Fe | CaCl2 |
Z | Na2SO4 | Cu(OH)2 | FeCl2 | CaCO3 |
A. A B. B C. C D. D