��Ŀ����
��17�֣�I��KNO3������ʯ����һ����Ҫ�Ļ���ԭ�ϣ���ũҵ����;ʮ�ֹ㷺��������һ��KNO3�Ʊ���������Ҫ���裺
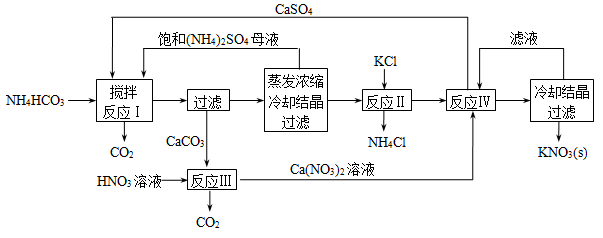
��1����Ӧ���У�CaSO4��NH4HCO3�����ʵ���֮��Ϊ1�U2���÷�Ӧ�Ļ�ѧ����ʽΪ ��
��2����Ӧ�����ڸ�̬�����ȵ������½��У����ȵ�Ŀ���� ���ӷ�Ӧ�����û�����з����CaSO4�ķ����� ������ȹ��ˡ�������ȴ���ˡ�����
��3�����鷴Ӧ������K2SO4���Ƿ����KCl�ķ����ǣ�ȡ����K2SO4��Ʒ�ܽ���ˮ�� ��
��4�����������У���ѭ�����õ����ʳ�(NH4)2SO4�⣬���� ���ѧʽ����
��5����������ŨKCl��Һ��ϣ�Ҳ�ɵõ�KNO3��ͬʱ���ɵ����������A������B��B����ԭ�ӷ��ӣ�B��O2��Ӧ����1�������ɫ����A��2�������ɫ����C��B�ķ���ʽΪ ��
II����1����ҵ�Ͽɲ��õ绯ѧ�ķ������N2H4��װ������ͼ��ʾ����ͨ�������� һ��Ϊ �����������������������NH3��Ӧ�ĵ缫��ӦʽΪ ��
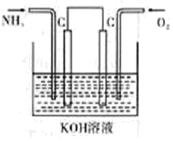
��2���£�N2H4�������ڴ�����ȼ�����ɵ�����ˮ��Ϊ�˳�������������� ���������ԭ���װ�ã��缫�����Ƕ��ʯī�缫�������Ϊ�ܹ�����H+�Ĺ������ʣ���д��������Ӧʽ ��

��1����Ӧ���У�CaSO4��NH4HCO3�����ʵ���֮��Ϊ1�U2���÷�Ӧ�Ļ�ѧ����ʽΪ ��
��2����Ӧ�����ڸ�̬�����ȵ������½��У����ȵ�Ŀ���� ���ӷ�Ӧ�����û�����з����CaSO4�ķ����� ������ȹ��ˡ�������ȴ���ˡ�����
��3�����鷴Ӧ������K2SO4���Ƿ����KCl�ķ����ǣ�ȡ����K2SO4��Ʒ�ܽ���ˮ�� ��
��4�����������У���ѭ�����õ����ʳ�(NH4)2SO4�⣬���� ���ѧʽ����
��5����������ŨKCl��Һ��ϣ�Ҳ�ɵõ�KNO3��ͬʱ���ɵ����������A������B��B����ԭ�ӷ��ӣ�B��O2��Ӧ����1�������ɫ����A��2�������ɫ����C��B�ķ���ʽΪ ��
II����1����ҵ�Ͽɲ��õ绯ѧ�ķ������N2H4��װ������ͼ��ʾ����ͨ�������� һ��Ϊ �����������������������NH3��Ӧ�ĵ缫��ӦʽΪ ��

��2���£�N2H4�������ڴ�����ȼ�����ɵ�����ˮ��Ϊ�˳�������������� ���������ԭ���װ�ã��缫�����Ƕ��ʯī�缫�������Ϊ�ܹ�����H+�Ĺ������ʣ���д��������Ӧʽ ��
I.��ÿ��2�֣���1��CaSO4��2NH4HCO3��CaCO3����(NH4)2SO4��H2O��CO2��
��2������NH4Cl��K2SO4���ӿ컯ѧ��Ӧ���� �� ���ȹ���
��3������Ba(NO3)2��Һ�����ٲ������������ã����ϲ���Һ�еμ�AgNO3��Һ�����г������ɣ�˵��K2SO4�л���KCl
��4��CaSO4��KNO3
��5��NOCl
II.��1��������1�֣�2NH3��2e?+2OH?=N2H4+2H2O��2�֣�
��2��N2H4��4e?=N2+4H+��2�֣�
��2������NH4Cl��K2SO4���ӿ컯ѧ��Ӧ���� �� ���ȹ���
��3������Ba(NO3)2��Һ�����ٲ������������ã����ϲ���Һ�еμ�AgNO3��Һ�����г������ɣ�˵��K2SO4�л���KCl
��4��CaSO4��KNO3
��5��NOCl
II.��1��������1�֣�2NH3��2e?+2OH?=N2H4+2H2O��2�֣�
��2��N2H4��4e?=N2+4H+��2�֣�
���������I.��1����������ͼ��֪��Ӧ����CaSO4��NH4HCO3����������CaCO3��(NH4)2SO4��CO2��H2O����ƽ�ɵû�ѧ����ʽ��CaSO4��2NH4HCO3��CaCO3����(NH4)2SO4��H2O��CO2��
��2����Ӧ������K2SO4��NH4Cl�������ڸ�̬�����ȵ������½��У������ڷ���NH4Cl��K2SO4���ӿ컯ѧ��Ӧ���� ��KNO3���ܽ�����¶�Ӱ����¶ȵ�ʱС��Ϊ�˷�ֹKNO3�ᾧ�����KNO3�IJ��ʣ�Ӧ���ȹ��ˡ�
��3��Ҫ����K2SO4���Ƿ����KCl����Ҫ�ų�SO42?�ĸ��ţ�������ȷ����Ϊ��ȡ����K2SO4��Ʒ�ܽ���ˮ������Ba(NO3)2��Һ�����ٲ������������ã����ϲ���Һ�еμ�AgNO3��Һ�����г������ɣ�˵��K2SO4�л���KCl����֮������KCl��
��4�����ݻ�ѧ����ͼ��CaSO4��KNO3��ѭ��ʹ�á�
��5��B����ԭ�ӷ��ӣ�B��O2��Ӧ����1�������A��2�������ɫ����C��CӦΪNO2������ɫ����ӦΪCl2����B�к���NԪ�غ�ClԪ�أ���Ӧ�ķ���ʽӦΪ2B+O2=Cl2+2NO2����֪B�ķ���ʽΪNOCl��
II.��1��NH3ת��ΪN2H4��NԪ�ػ��ϼ����ߣ�NH3����ԭ����������������������ͨ�������ĵ缫Ϊ������NH3ʧȥ����ת��ΪN2H4�����ݻ��ϼ۵ı仯��ƽ�ɵõ缫����ʽ��2NH3��2e?+2OH?=N2H4+2H2O
��2���£�N2H4�������ڴ�����ȼ�����ɵ�����ˮ�������Ϊ�ܹ�����H+�Ĺ������ʣ����Ը�����N2H4ʧ��������N2��H+���缫����ʽΪ��N2H4��4e?=N2+4H+

��ϰ��ϵ�д�
�����Ŀ
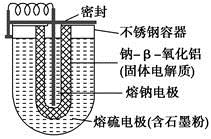
 Cd+2NiO(OH)+2H2O �ɴ˿�֪���õ�طŵ�ʱ�ĸ��������� �� ��
Cd+2NiO(OH)+2H2O �ɴ˿�֪���õ�طŵ�ʱ�ĸ��������� �� ��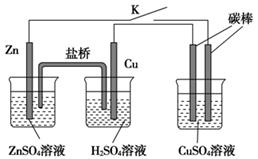
 Na2Sn������˵������ȷ����
Na2Sn������˵������ȷ����