��Ŀ����
һ���¶��£���ͬ������ĸ����������зֱ���������ĸ�ƽ�⣺
��N2(g)+3H2(g) 2NH3(g) K1
2NH3(g) K1
��H2(g)+I2(g) 2HI(g) K2
2HI(g) K2
��2NO2(g) N2O4(g) K3
N2O4(g) K3
��C(s)+H2O(g) CO(g)+H2(g) K4
CO(g)+H2(g) K4
��������и��⣺
��1��д����Ӧ�ܵ�ƽ�ⳣ���ı���ʽK4=____________��
��2��������ͬ�¶��µ�����ƽ�⣺
�� 2N2(g)+6H2(g) 4NH3(g) K5
4NH3(g) K5
��2HI(g) H2(g)+I2(g) K6
H2(g)+I2(g) K6
��K5=__ ��K6=_ ������K1��K2��K3��K4��ʾ��
��3����ƽ�����NO2���������Ϊa��ijʱ���ټ���һ������N2O4����ʱ�ԣ����� �ԣ��棩���ٴδﵽƽ���NO2��������� a�����������������������
��ϰ��ϵ�д�
 һ����ʦ�����Ծ�ϵ�д�
һ����ʦ�����Ծ�ϵ�д�
�����Ŀ


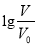 �ı仯��ͼ��ʾ����������������ǣ� ��
�ı仯��ͼ��ʾ����������������ǣ� ��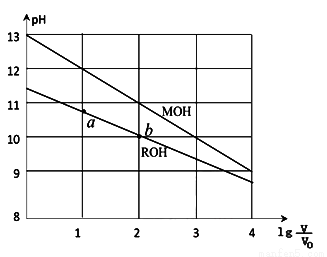
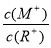 ����
���� H2O+CO2��
H2O+CO2�� ͭм������������Һ�У�Cu��2Ag+
ͭм������������Һ�У�Cu��2Ag+