��Ŀ����
����TiO2��Ϳ�ϡ��������ױƷ���������ż���㷺��Ӧ�á�
��1����ҵ�϶������ѵ��Ʊ��ǣ� ���Ͽ�Ƭ��
�����۵�SiCl4 ��70 �� �� TiCl4 ��25 �棻���ʷе�SiCl457.6 �桢TiCl4136.5 ��
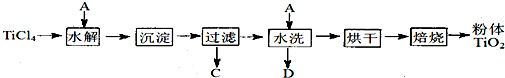
�������Ľ��ʯ����Ҫ�ɷ�TiO2����Ҫ����SiO2����̼�ۻ��װ���Ȼ�¯�У��ڸ�����ͨ��Cl2��Ӧ���Ƶû���SiCl4���ʵ�TiCl4��
��SiCl4���룬�õ�������TiCl4��
����TiCl4�м�ˮ�����ȣ�ˮ��õ�����TiO2��xH2O��
���������ˡ�ˮϴ��ȥ���е�Cl�����ٺ�ɡ����ճ�ȥˮ�ֵõ�����TiO2 ��
���ڳ����·���TiCl4��SiCl4����ȡ�IJ��������� ��
�ڢ��з�Ӧ�Ļ�ѧ����ʽ�� ��
�� ����TiO2��x H2O��Cl���Ƿ����ķ�����______________________________��
��2��TiO2���ӵĴ�С�������ִ����������ⶨ������������ԭ�ζ����ɲⶨTiO2������������һ�������£���TiO2�ܽⲢ��ԭΪTi3+ ������KSCN��Һ��ָʾ������NH4Fe(SO4)2����Һ�ζ�Ti3+��ȫ������Ti4+���ش��������⣺
�����п����ڲⶨTiO2���Ӵ�С�ķ�����_____________________������ĸ���ţ���
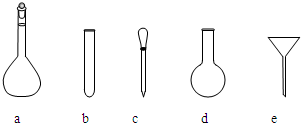
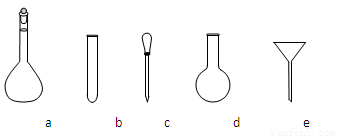
a.�˴Ź��� b.������� c.���� d.�����������
������NH4Fe(SO4)2����Һʱ��ʹ�õ���������ƽ��ҩ�ס����������ձ�����Ͳ�⣬����Ҫ��ͼ�е�_____������ĸ���ţ���
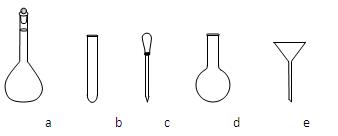
�۵ζ��յ��������___________________________________________________��
�ܵζ�����ʱ����ȡTiO2��Ħ������ΪM g��mol��1������w g������c mol/L NH4Fe(SO4)2����ҺV mL����TiO2������������ʽΪ_________________________��
���������Ʊ���Һ�����У��ձ��е�NH4Fe(SO4)2��Һ��������������TiO2���������ⶨ�����Ӱ��_________________________�����ƫ�ߡ�����ƫ�͡�����Ӱ�족��
��1�������� ��TiCl4+(2+x) H2O= TiO2��xH2O+4HCl
�ۼ�������Ƿ�ϴ���ķ����ǣ�ȡ����ϴ��Һ������ϴ��Һ���ܽ�������Ƿ���Cl����
��2���ٺ˴Ź��������ڲ��л����к��ж�������ԭ�ӣ�����������л��ﺬ�к��ֻ�ѧ�������ţ����������ڲ��л�����Է���������������������Թ۲쵽���Ĵ�С�� d ��a c ����Һ��ɺ�ɫ��30���ڲ���ɫ �ܸ��ݵ�ʧ�����غ㣬�У�1Ti3+��1Fe3+,��n(Fe3+)= n(Ti3+)= n(TiO2)=cV��10-3mol������������Ϊ![]() ��
��
��NH4Fe(SO4)2��Һ�����������ʵ���Ũ�ȼ�С�����ĵ�������ٷֺ���ƫ��
����:
��

 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�
 TiO2?xH2O��+4HCl
TiO2?xH2O��+4HCl