��Ŀ����
����TiO2��Ϳ�ϡ��������ױƷ���������ż���㷺��Ӧ�ã��Ʊ�����TiO2�ķ���֮һ��TiCl4ˮ������TiO2?xH2O�������ˡ�ˮϴ��ȥ���е�Cl-���ٺ�ɡ����ճ�ȥˮ�ֵõ�����TiO2����������ԭ�ζ����ⶨTiO2�����������������£�������һ�������£���TiO2�ܽⲢת��ΪTi3+������NH4Fe��SO4��2����Һ�ζ�Ti3+��ȫ��ת����Ti4+��
��ش��������⣺
��1��TiCl4ˮ������TiO2?xH2O�Ļ�ѧ����ʽΪ
��2������TiO2?xH2O��Cl-�Ƿ����ķ�����
��3������NH4Fe��SO4��2����Һʱ������һ����H2SO4��Ŀ����
��4���õζ��������õ���ָʾ����
��5���ζ�����ʱ����ȡTiO2��Ħ������ΪM g?mol-1������w g������c mol?L-1NH4Fe��SO4��2����ҺV mL����TiO2��������Ϊ
��
%
��
%��
��ش��������⣺

��1��TiCl4ˮ������TiO2?xH2O�Ļ�ѧ����ʽΪ
TiCl4+��x+2��H2O=TiO2?xH2O��+4HCl
TiCl4+��x+2��H2O=TiO2?xH2O��+4HCl
����2������TiO2?xH2O��Cl-�Ƿ����ķ�����
ȡ�������һ��ˮϴҺ���μ�AgNO3��Һ����������ɫ������˵��Cl-�ѳ���
ȡ�������һ��ˮϴҺ���μ�AgNO3��Һ����������ɫ������˵��Cl-�ѳ���
����3������NH4Fe��SO4��2����Һʱ������һ����H2SO4��Ŀ����
����NH4Fe��SO4��2ˮ��
����NH4Fe��SO4��2ˮ��
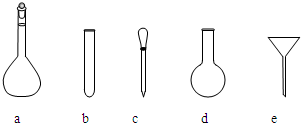
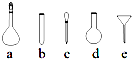
��������Һʹ�õ���������ƽ��ҩ�ס����������ձ�����Ͳ�⣬����Ҫ��ͼ�е�ac
ac
������ĸ���ţ�����4���õζ��������õ���ָʾ����
KSCN��Һ
KSCN��Һ
���ζ��յ����������Һ��Ϊ��ɫ���Ұ���Ӳ���ɫ
��Һ��Ϊ��ɫ���Ұ���Ӳ���ɫ
��5���ζ�����ʱ����ȡTiO2��Ħ������ΪM g?mol-1������w g������c mol?L-1NH4Fe��SO4��2����ҺV mL����TiO2��������Ϊ
| cVM |
| 1000W |
| cVM |
| 10W |
| cVM |
| 1000W |
| cVM |
| 10W |
��������1�����ݷ�Ӧ����������������غ㶨������д��ѧ����ʽ��
��2����Һ������TiO2?xH2O������������н�ǿ��������������������Һ�е�Cl-���ӣ���ͨ������Cl-���ӵķ�����������Ƿ�ϴ����
��3����������ˮ�⣬������Һʱ�ȼ�һЩ���ᣬʹ��Һ�����ԣ�����NH4Fe��SO4��2ˮ�⣬����һ�����ʵ���Ũ�ȵ���Һ����Ҫ�������ɸ��ݾ���IJ�������ѡ��
��4����Ϊ����KSCN��ָʾ�����յ�ʱNH4Fe��SO4��2���ٷ�Ӧ������Ѫ��ɫ��Fe��SCN��3
��5�����ݵ�ʧ�����غ㣺1Ti3+��1Fe3+����n��Fe3+��=n��Ti3+��=n��TiO2��=cV��10-3mol���Դ˽��м��㣮
��2����Һ������TiO2?xH2O������������н�ǿ��������������������Һ�е�Cl-���ӣ���ͨ������Cl-���ӵķ�����������Ƿ�ϴ����
��3����������ˮ�⣬������Һʱ�ȼ�һЩ���ᣬʹ��Һ�����ԣ�����NH4Fe��SO4��2ˮ�⣬����һ�����ʵ���Ũ�ȵ���Һ����Ҫ�������ɸ��ݾ���IJ�������ѡ��
��4����Ϊ����KSCN��ָʾ�����յ�ʱNH4Fe��SO4��2���ٷ�Ӧ������Ѫ��ɫ��Fe��SCN��3
��5�����ݵ�ʧ�����غ㣺1Ti3+��1Fe3+����n��Fe3+��=n��Ti3+��=n��TiO2��=cV��10-3mol���Դ˽��м��㣮
�����������1����TiCl4��ϵ��Ϊ1������Ԫ���غ㣬TiO2?xH2O��ϵ��Ϊ1��HCl��ϵ��Ϊ4���ٸ���OԪ���غ㣬��֪H2O��ϵ��Ϊ��2+x�����ʴ�Ϊ��TiCl4+��x+2��H2O=TiO2?xH2O��+4HCl��
��2����Һ������TiO2?xH2O������������н�ǿ��������������������Һ�е�Cl-���ӣ���ͨ������Cl-���ӵķ�����������Ƿ�ϴ������������Ƿ�ϴ���ķ����ǣ�ȡ����ϴ��Һ��������Һ���ܽ�������Ƿ��ڣ��ʴ�Ϊ��ȡ�������һ��ˮϴҺ���μ�AgNO3��Һ����������ɫ������˵��Cl-�ѳ�����
��3����������ˮ�⣬������Һʱ�ȼ�һЩ���ᣬʹ��Һ�����ԣ�����NH4Fe��SO4��2ˮ�⣬����һ�����ʵ���Ũ�ȵ���Һ��������Һʹ�õ���������ƽ��ҩ�ס����������ձ�����Ͳ������ƿ�ͽ�ͷ�ιܵȣ��ʴ�Ϊ������NH4Fe��SO4��2ˮ�⣬a c��
��4����Ϊ����KSCN��ָʾ�����յ�ʱNH4Fe��SO4��2���ٷ�Ӧ������Ѫ��ɫ��Fe��SCN��3���ʴ�Ϊ��KSCN��Һ����Һ��Ϊ��ɫ���Ұ���Ӳ���ɫ��
��5�����ݵ�ʧ�����غ㣬�У�1Ti3+��1Fe3+����n��Fe3+��=n��Ti3+��=n��TiO2��=cV��10-3mol������������Ϊ
��
%��
�ʴ�Ϊ��
��
%��
��2����Һ������TiO2?xH2O������������н�ǿ��������������������Һ�е�Cl-���ӣ���ͨ������Cl-���ӵķ�����������Ƿ�ϴ������������Ƿ�ϴ���ķ����ǣ�ȡ����ϴ��Һ��������Һ���ܽ�������Ƿ��ڣ��ʴ�Ϊ��ȡ�������һ��ˮϴҺ���μ�AgNO3��Һ����������ɫ������˵��Cl-�ѳ�����
��3����������ˮ�⣬������Һʱ�ȼ�һЩ���ᣬʹ��Һ�����ԣ�����NH4Fe��SO4��2ˮ�⣬����һ�����ʵ���Ũ�ȵ���Һ��������Һʹ�õ���������ƽ��ҩ�ס����������ձ�����Ͳ������ƿ�ͽ�ͷ�ιܵȣ��ʴ�Ϊ������NH4Fe��SO4��2ˮ�⣬a c��
��4����Ϊ����KSCN��ָʾ�����յ�ʱNH4Fe��SO4��2���ٷ�Ӧ������Ѫ��ɫ��Fe��SCN��3���ʴ�Ϊ��KSCN��Һ����Һ��Ϊ��ɫ���Ұ���Ӳ���ɫ��
��5�����ݵ�ʧ�����غ㣬�У�1Ti3+��1Fe3+����n��Fe3+��=n��Ti3+��=n��TiO2��=cV��10-3mol������������Ϊ
| cVM |
| 1000W |
| cVM |
| 10W |
�ʴ�Ϊ��
| cVM |
| 1000W |
| cVM |
| 10W |
������������Χ�ƻ�ѧʵ����Ƶ��ۺ������⣬��Ҫ�����У���ѧ����ʽ��ƽ��������ϴ�ӣ�����Һ�����ơ��ζ����������������ļ���ȣ�

��ϰ��ϵ�д�
 ���ĺ����Ͼ�������ϵ�д�
���ĺ����Ͼ�������ϵ�д�
�����Ŀ
 TiO2?xH2O��+4HCl
TiO2?xH2O��+4HCl