��Ŀ����
��8�֣��������γɶ������ӣ���N3����NH2����N3����NH4+��N2H5+��N2H62+�ȣ���֪N2H5+��N2H62+�������Է��ӽ�������γɵģ�������NH4+������������� NH4+�����ʡ�
��1��NH4+�ĵ���ʽΪ ��N���� �ӻ���ʽ��NH4+���ӿռ乹��Ϊ ������Ϊ ��
��2��д�������ɶ��ԭ����ɵĺ�����N3���۵�������ͬ�����ʵĻ�ѧʽ ��
��3���ȵ�������������������ƵĽṹ����Ԥ��N3���Ĺ��� ��
��1��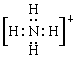 sp3 ������ 109��28�� (2) CO2��N2O ��3��ֱ����
sp3 ������ 109��28�� (2) CO2��N2O ��3��ֱ����
����

��ϰ��ϵ�д�
�����Ŀ

