��Ŀ����
�������γɶ������ӣ���N3-��NH2-��N3-��NH4+��N2H5+��N2H62+�ȣ���֪N2H5+��N2H62+�������Է��ӽ�������γɵģ�����
��NH4+������������� NH4+�����ʣ�
��1��д��N2H62+�ڼ�����Һ�з�Ӧ�����ӷ���ʽ
��2��NH2-�ĵ���ʽΪ
 ��
��
��3��N3-��
��4��д�������ɶ��ԭ����ɵĺ�����N3-��������ͬ�����ʵĻ�ѧʽ
��5���ȵ��������������������ƵĽṹ����Ԥ��N3-�Ĺ���
��6���ݱ�����������ѧ�ҿ���?����˹����1998��11�ºϳ���һ����Ϊ��N5�������ʣ���������м�ǿ�ı�ը�ԣ��ֳ�Ϊ������ը����������Ϊֹ�����Ƕ����Ľṹ�в������ֻ֪����N5��ʵ�����Ǵ�����ɵķ�����Ƭ����ṹ�ǶԳƵģ�5��N�ų�V�Σ����5��N��Ϻﵽ8���ӽṹ���Һ���2��N��N������N5��������Ƭ���������
��NH4+������������� NH4+�����ʣ�
��1��д��N2H62+�ڼ�����Һ�з�Ӧ�����ӷ���ʽ
N2H62++2OH-=N2H4+2H2O
N2H62++2OH-=N2H4+2H2O
����2��NH2-�ĵ���ʽΪ


��3��N3-��
22
22
�����ӣ���4��д�������ɶ��ԭ����ɵĺ�����N3-��������ͬ�����ʵĻ�ѧʽ
N2O��CO2
N2O��CO2
����5���ȵ��������������������ƵĽṹ����Ԥ��N3-�Ĺ���
ֱ����
ֱ����
����6���ݱ�����������ѧ�ҿ���?����˹����1998��11�ºϳ���һ����Ϊ��N5�������ʣ���������м�ǿ�ı�ը�ԣ��ֳ�Ϊ������ը����������Ϊֹ�����Ƕ����Ľṹ�в������ֻ֪����N5��ʵ�����Ǵ�����ɵķ�����Ƭ����ṹ�ǶԳƵģ�5��N�ų�V�Σ����5��N��Ϻﵽ8���ӽṹ���Һ���2��N��N������N5��������Ƭ���������
һ����λ�����
һ����λ�����
����������1����N2H62+��N�Ļ��ϼ�Ϊ+4�ۣ�N2H62+�������Է���
N2H4 ���2�������γɵģ��൱�ڹ�N2H62+�൱�ڶ�Ԫ�ᣬ������Һ�з�Ӧ�����ӷ���ʽΪN2H62++2OH-=N2H4+2H2O��
��2��NH2-�ĵ���ʽΪ��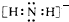 ��
��
��3��1��N����7�����ӣ���N3-��22�����ӣ�
��4������22�����ӵĶ�ԭ��������N2O��CO2��CNO-��BeF2��CaH2��C3H4�ȣ�
��5��CO2Ϊֱ���ͷ��ӣ��ʿ���ΪN3-�Ĺ���Ϊֱ���ͣ�
��6��N5�ṹ�ǶԳƵģ�5��N�ų�V�Σ�5��N��Ϻﵽ8���ӽṹ���Һ���2��N��N�������������ĽṹΪ��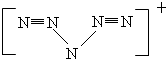 ���ʡ�N5����һ����λ����ɣ�
���ʡ�N5����һ����λ����ɣ�
��2��NH2-�ĵ���ʽΪ��
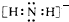 ��
����3��1��N����7�����ӣ���N3-��22�����ӣ�
��4������22�����ӵĶ�ԭ��������N2O��CO2��CNO-��BeF2��CaH2��C3H4�ȣ�
��5��CO2Ϊֱ���ͷ��ӣ��ʿ���ΪN3-�Ĺ���Ϊֱ���ͣ�
��6��N5�ṹ�ǶԳƵģ�5��N�ų�V�Σ�5��N��Ϻﵽ8���ӽṹ���Һ���2��N��N�������������ĽṹΪ��
 ���ʡ�N5����һ����λ����ɣ�
���ʡ�N5����һ����λ����ɣ�����⣺��1����N2H62+��N�Ļ��ϼ�Ϊ+4�ۣ�N2H62+�������Է���N2H4���2�������γɵģ���N2H62+�൱�ڶ�Ԫ�ᣬ���ڼ�����Һ�з�Ӧ�����ӷ���ʽΪN2H62++2OH-=N2H4+2H2O���ʴ�Ϊ��N2H62++2OH-=N2H4+2H2O��
��2��NH2-�ĵ���ʽΪ��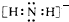 ���ʴ�Ϊ��
���ʴ�Ϊ��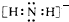 ��
��
��3��1��N����7�����ӣ���N3-��22�����ӣ��ʴ�Ϊ��22��
��4������22�����ӵĶ�ԭ��������N2O��CO2��CNO-��BeF2��CaH2��C3H4�ȣ��ʴ�Ϊ��N2O��CO2��
��5��CO2Ϊֱ���ͷ��ӣ��ʿ���ΪN3-�Ĺ���Ϊֱ���ͣ��ʴ�Ϊ��ֱ���ͣ�
��6��N5�ṹ�ǶԳƵģ�5��N�ų�V�Σ�5��N��Ϻﵽ8���ӽṹ���Һ���2��N��N�������������ĽṹΪ�� ���ʡ�N5����һ����λ����ɣ��ʴ�Ϊ��һ����λ����ɣ�
���ʡ�N5����һ����λ����ɣ��ʴ�Ϊ��һ����λ����ɣ�
��2��NH2-�ĵ���ʽΪ��
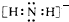 ���ʴ�Ϊ��
���ʴ�Ϊ��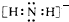 ��
����3��1��N����7�����ӣ���N3-��22�����ӣ��ʴ�Ϊ��22��
��4������22�����ӵĶ�ԭ��������N2O��CO2��CNO-��BeF2��CaH2��C3H4�ȣ��ʴ�Ϊ��N2O��CO2��
��5��CO2Ϊֱ���ͷ��ӣ��ʿ���ΪN3-�Ĺ���Ϊֱ���ͣ��ʴ�Ϊ��ֱ���ͣ�
��6��N5�ṹ�ǶԳƵģ�5��N�ų�V�Σ�5��N��Ϻﵽ8���ӽṹ���Һ���2��N��N�������������ĽṹΪ��
 ���ʡ�N5����һ����λ����ɣ��ʴ�Ϊ��һ����λ����ɣ�
���ʡ�N5����һ����λ����ɣ��ʴ�Ϊ��һ����λ����ɣ������������ص㿼���˵���ʽ����д�Լ��ȵ����������ʣ�����ԭ�ӽṹ����ӹ��͵ķ�����ע�����ԭ�ӵĵ����Ų�����Ŀ�ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
