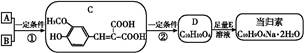
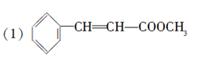��Ŀ����
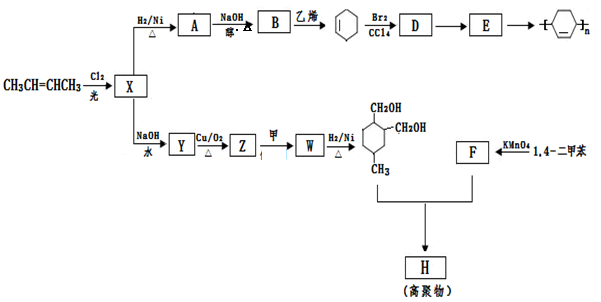
���۷��ɸĽ��л��߷��ӻ���������ʣ��߷��Ӿۺ���P�ĺϳ�·�����£�
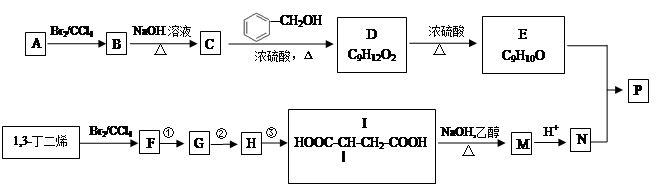
��1��A�Ľṹ��ʽΪ________________
��2��C������Ϊ
��3��I��F���١��ۺϳɣ�F����ʹ��ˮ��ɫ.
a���ٵĻ�ѧ����ʽ��
b���ڵķ�Ӧ�Լ���
c���۵ķ�Ӧ������
��4������˵����ȷ����
a��C����ˮ����������
b��A��1,3-����ϩ��Ϊͬϵ��
c����I����Mʱ��1mol�������3molNaOH
d��N������˳���칹��
��5���߾���P����ˮ�ԣ�����E�γɵľۺ���__________���ǿ��������.
��6��E��N�������ʵ���֮��Ϊ1:1������������P��P�Ľṹ��ʽΪ
��7��E�ж���ͬ���칹�壬���������������칹���� �֣�������˳���칹����д�����е�һ��
a��������ֻ��һ�ֻ�״�ṹ
b��������������ȡ����
c��1mol���л�������ˮ��Ӧʱ������4molBr2

��1��A�Ľṹ��ʽΪ________________
��2��C������Ϊ
��3��I��F���١��ۺϳɣ�F����ʹ��ˮ��ɫ.
a���ٵĻ�ѧ����ʽ��
b���ڵķ�Ӧ�Լ���
c���۵ķ�Ӧ������
��4������˵����ȷ����
a��C����ˮ����������
b��A��1,3-����ϩ��Ϊͬϵ��
c����I����Mʱ��1mol�������3molNaOH
d��N������˳���칹��
��5���߾���P����ˮ�ԣ�����E�γɵľۺ���__________���ǿ��������.
��6��E��N�������ʵ���֮��Ϊ1:1������������P��P�Ľṹ��ʽΪ
��7��E�ж���ͬ���칹�壬���������������칹���� �֣�������˳���칹����д�����е�һ��
a��������ֻ��һ�ֻ�״�ṹ
b��������������ȡ����
c��1mol���л�������ˮ��Ӧʱ������4molBr2
��1��CH2=CH2
��2���Ҷ���
(3)a��BrCH2CH=CHCH2Br+ 2NaOH
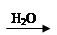 HOCH2CH=CHCH2OH+ 2NaBr
HOCH2CH=CHCH2OH+ 2NaBrb���ӳɷ�Ӧ
c��������Ӧ
��4��a��c
��5��ǿ
��6��
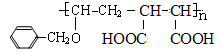
(7 )
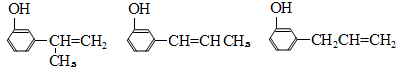
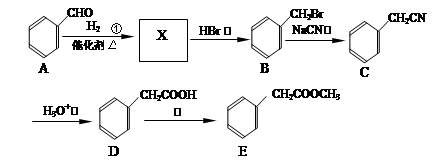
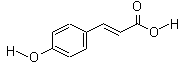
���������������������֪A�����ӳɡ�ˮ�⡢��ˮ���ѣ���֪����������ˮ��ȥ����E������A��B��C��D��E�ֱ�ΪCH2=CH2��CH2BrCH2Br��CH2OHCH2OH��
 ��
��
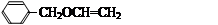
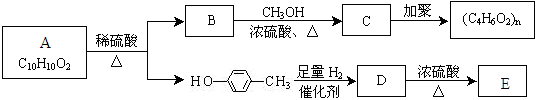
������������������̣�1,3-����ϩ����1,4-�ӳɣ�ˮ�⡢�ӳ�HCl������̼̼˫��������������֪����������ȥ�Ȳ���õ�M������F��G��H��M��N�ֱ�ΪBrCH2CH=CHCH2Br��HOCH2CH=CHCH2OH��HOCH2CH2CHClCH2OH��NaOOCCH=CHCOONa��HOOCCH=CHCOOH��(3)F ����̼̼˫�����ٵĻ�ѧ����ʽ��BrCH2CH=CHCH2Br+ 2NaOH
 HOCH2CH=CHCH2OH+ 2NaBr����4�� a��CΪ�Ҷ�������ˮ���ܣ���ȷ��b��AΪ��ϩ�ǵ�ϩ����1,3-����ϩ�Ƕ�ϩ������������Ŀ��ͬ������ͬϵ����� c.I����2���Ȼ���1����ԭ��ˮ������HCl����1molI�������3molNaOH����ȷ��d.N̼̼˫�����˵�̼ԭ���������ֲ�ͬ��ԭ�ӣ��ʴ���˳���칹�壬����5��P�к�����ˮ���Ȼ���������ˮ����ǿ������ˮ����E�γɵľۺ����6��N��F�ۺϵõ�
HOCH2CH=CHCH2OH+ 2NaBr����4�� a��CΪ�Ҷ�������ˮ���ܣ���ȷ��b��AΪ��ϩ�ǵ�ϩ����1,3-����ϩ�Ƕ�ϩ������������Ŀ��ͬ������ͬϵ����� c.I����2���Ȼ���1����ԭ��ˮ������HCl����1molI�������3molNaOH����ȷ��d.N̼̼˫�����˵�̼ԭ���������ֲ�ͬ��ԭ�ӣ��ʴ���˳���칹�壬����5��P�к�����ˮ���Ȼ���������ˮ����ǿ������ˮ����E�γɵľۺ����6��N��F�ۺϵõ�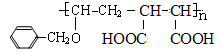 ��
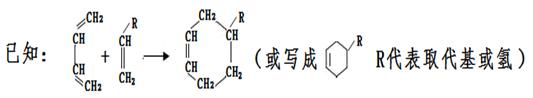
����7�����ǵ���ͬ���칹���о߱����������������к��з��ǻ�����Ӧ�����ڶ�λ����λ�ñ���ȡ������Ӧ��1��̼̼˫������������3�֣�
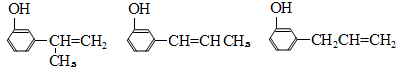

��ϰ��ϵ�д�
 ȫ�ܲ����ĩС״Ԫϵ�д�
ȫ�ܲ����ĩС״Ԫϵ�д�
�����Ŀ
 RCH
RCH C��COOH��2+H2O��RCH
C��COOH��2+H2O��RCH RCH
RCH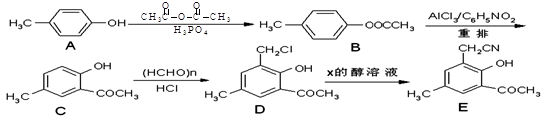
 R-COOH��E������������ˮ���IJ�����һ�������¿�����F��C11H10O3����д��F�Ľṹ��ʽ�� ��
R-COOH��E������������ˮ���IJ�����һ�������¿�����F��C11H10O3����д��F�Ľṹ��ʽ�� ��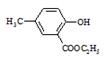 �ĺϳ�·������ͼ��
�ĺϳ�·������ͼ�� CH3CH2OH
CH3CH2OH  H2C��CH2 BrH2C��CH2Br
H2C��CH2 BrH2C��CH2Br  �������׳��Ჴ������������������������ù���к�ǿ���������á���������£�
�������׳��Ჴ������������������������ù���к�ǿ���������á���������£�