��Ŀ����
����Ŀ��[��ѧ��ѡ��2����ѧ�뼼��]
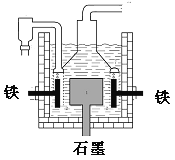
��ҵ��ͭ�ķ������ɻ�ͭ������Ҫ�ɷ���CuFeS2,���ʲ���ͭԪ�������ƾ�ͭ�Ĺ�������ʾ��ͼ��ͼ��
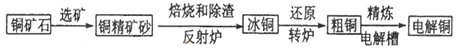
��1������ͭ����ͭ����������Ϊ0.4������1�ִ�ͭ��������Ҫ���ֻ�ͭ��____________�֡�
��2�����ڷ���¯�У���ͭ����ɰ��ʯӢɰ��ϼ��ȵ�1000�����ң���ͭ���������Ӧ����Cu��Fe�ĵͼ����_____________________���Ҳ���Fe������ת��ΪFe�ĵͼ������
��3�����ӻ��������ͳ������ԭ�ϽǶȿ���δ��������ò�����������___________��
��4������ת¯�У���ͭ�е�Cu2S�ȱ�������Cu2O��,���ɵ�Cu2O����Cu2S��Ӧ��������Ӧ���Ȼ�ѧ����ʽΪ����2Cu2S��s��+3O2��g���T2Cu2O��s����+2SO2��g����H=-768.2kJ.mol-1
��2Cu2O��s��+Cu2S��s���T6Cu��s��+SO2��g����H=+116.0kJ.mol-1
��ӦCu2Sת��Ϊͭ���Ȼ�ѧ����ʽ��_________________________��
��3molCu2S��һ�����Ŀ����������������������Ϊ4:1�����ܱ������г�ַ�Ӧ�����������Ӧ����ȫ��Cu2S��ȫ���뷴Ӧ�����õ�����ͭ3mol������������SO2���������Ϊ_____________��
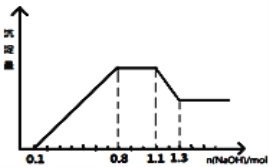
��5������⾫��ͭ���������ҺΪ______________�����һ��ʱ��������Һ��Ũ��_____________������������������������������������
��6�������ڷ�ӦCu+H2O2+H2SO4=CuSO4+2H2O�����Ʊ�CuSO4�������÷�Ӧ���Ϊԭ��أ��������缫��ӦʽΪ__________________��
���𰸡���1����2.5t��2����
��2����2CuFeS2+O2![]() Cu2S+2FeS+SO2��2����
Cu2S+2FeS+SO2��2����
��3�����ð�ˮ���������е�SO2������NH4��2SO3��NH4HSO3���������ɵ���NH4��2SO3��NH4HSO3�����ᷴӦ����SO2���ռ�SO2��������H2SO4
��4����Cu2S��s��+O2��g��=2Cu��s��+SO2��g����H=-217.4kJ.mol-1��2����16.7%��2����
��5����CuSO4Cu��NO3��2��1����,������2������6����H2O2+2H++2e-=2H2O��2����
��������
�����������1����ͭ����ͭ����������Ϊ0.4������1�ִ�ͭ��������Ҫ���ֻ�ͭ��������Ϊ![]() ��
��
��2��.�ڷ���¯�У���ͭ����ɰ��ʯӢɰ��ϼ��ȵ�1000�����ң���ͭ���������Ӧ����Cu��Fe�ĵͼ����������������ͭ�����������Ҳ���Fe������ת��ΪFe�ĵͼ���������������SO2���ɣ���Ӧ�ķ���ʽΪ2CuFeS2+O2![]() Cu2S+2FeS+SO2��
Cu2S+2FeS+SO2��
��3��.��������Ҫ�ɷ��Ƕ����������ӻ��������ͳ������ԭ�ϽǶȿ������ķ������ð�ˮ���������е�SO2������NH4��2SO3��NH4HSO3���������ɵ���NH4��2SO3��NH4HSO3��
��4����֪����2Cu2S��s��+3O2��g���T2Cu2O��s����+2SO2��g����H=-768.2kJ.mol-1
��2Cu2O��s��+Cu2S��s���T6Cu��s��+SO2��g����H=+116.0kJ.mol-1
�����ݸ�˹���ɿ�֪����+������3���õ���ӦCu2Sת��Ϊͭ���Ȼ�ѧ����ʽΪCu2S��s��+O2��g����2Cu��s��+SO2��g����H=-217.4kJ.mol-1��
��3molCu2S��һ�����Ŀ����������������������Ϊ4:1�����ܱ������г�ַ�Ӧ�����������Ӧ����ȫ��Cu2S��ȫ���뷴Ӧ�����õ�����ͭ3mol����
2Cu2S+3O2��2Cu2O+2SO2
2.5mol 3.75mol 2.5mol 2.5mol
Cu2S+2Cu2O��6Cu+SO2��
0.5mol 1mol 3mol 0.5mol
��Ӧ��������Ϊ3.75mol�����������ʵ�����������4�������������ʵ���Ϊ15mol�����ɵĶ�������Ϊ3mol�������������еĶ���������������![]() ��100%=16.7%��
��100%=16.7%��
��5����⾫��ͭʱ�������ҺΪCuSO4�����ڴ�ͭ�к������ʣ�����Ҳʧȥ���ӣ���˵��һ��ʱ��������Һ��Ũ�ȼ��١�
��6�����ڷ�ӦCu+H2O2+H2SO4=CuSO4+2H2O�����Ʊ�CuSO4�������÷�Ӧ���Ϊԭ��أ��������缫��˫��ˮ�õ����ӣ��������缫��ӦʽΪH2O2+2H++2e-=2H2O��

 ������������ϵ�д�
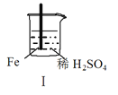
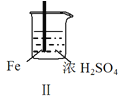
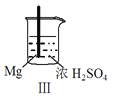
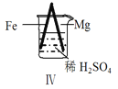
������������ϵ�д�����Ŀ�� Fe��Mg��H2SO4��Ӧ��ʵ�����£�
ʵ�� |
|
|
|
|
���� | Fe�������������ɫ���� | Fe����������ݺ�Ѹ��ֹͣ | Mg����Ѹ�ٲ����������� | Fe�����д������ݣ�Mg�������������� |
��������ʵ��˵�������������� ��
A��I�в��������ԭ���ǣ�Fe + 2H��=Fe2��+ H2��
B��ȡ�����е���������CuSO4��Һ������������ɫ����
C����������˵��Mg��ŨH2SO4��û���ۻ�
D����������˵��Mg�Ľ����Ա�Feǿ