��Ŀ����
����Ŀ���̷���![]() ��������ȱ����ƶѪ����Чҩ��ij��ѧ��ȤС����̷����������µ�̽����
��������ȱ����ƶѪ����Чҩ��ij��ѧ��ȤС����̷����������µ�̽����
I .���Ʊ���Ʒ��
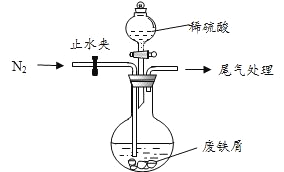
��С���ɷ���м������������ͭ�������������ʣ�������ͼ��ʾװ���Ʊ�![]() ���壬�������£�
���壬�������£�
��1��Ԥ�������Ƚ�����м���뵽����![]() ��Һ��ϴ�ӣ�Ŀ����_____��Ȼ����м��ˮϴ��2��3 �顣
��Һ��ϴ�ӣ�Ŀ����_____��Ȼ����м��ˮϴ��2��3 �顣
��2����ϴ�Ӻ�ķ���м���뵽Բ����ƿ�У�������ͨ��![]() ��
��![]() ��������________��
��������________��
��3���ټ�������ϡ���ᣬ�����¶� 50��~80��֮�䣬��ַ�Ӧ��Բ����ƿ��ʣ��Ĺ���Ϊ_____________��
��4����ȡ��Ʒ�������裨3���з�Ӧ��Ļ�����м�����������ˮ�����ȹ��ˣ� ____________��
�˳����壬��������ˮϴ�� 2��3 �Σ�������ֽ���������ɣ��ܱձ��档
II.���ⶨ![]() ������
������
��1����ȡ������Ʒ 10.0g������������ϡ�����У���� 100mL ��Һ����Ҫ����������ƽ����ͷ�ιܡ� �ձ�����Ͳ�⣬����Ҫ�������У����������ƣ�____________________��_______________________��
��2��ȷ��ȡ 25mL ��Һ������ƿ�У��� 0.1000mol/L ![]() ����Һ�ζ�����ζ��յ���жϷ�����________________________��
����Һ�ζ�����ζ��յ���жϷ�����________________________��
��3����ͬ���ķ����ζ� 3 �Σ�ƽ������ 10.00mL ��Һ������Ʒ��![]() ����������Ϊ____________������֪ Mr(
����������Ϊ____________������֪ Mr(![]() )=278��
)=278��
��4�����������ƫС����������ڶ���ʱ_________________(����ӡ������ӡ�)������
���𰸡� ϴȥ��м��������� �ų�װ���еĿ��������� Cu ��ȴ�ᾧ 100ml ����ƿ ������ �����һ�α�Һ����ʱ����Һ��Ϊ��ɫ���� 30s ���ֲ��� 55.6% ����
�����������⿼��ʵ�鷽����������ۣ�I.��1��̼������Һ�Լ��ԣ���֬�ڼ���������ˮ��ɿ�����ˮ�����ʣ����Ŀ���dz�ȥ��м��������ۣ���2����Ϊ�Ʊ�FeSO4��7H2O������Fe2�����ױ�����������Fe3������˷�Ӧǰ��ͨ��һ��ʱ��ĵ�������Ŀ�����ų�װ���е��������������3������м�ɷ��������ʡ�����ͭ�����������������ᣬ������Ӧ��Fe2O3��6H��=2Fe3����3H2O��CuO��2H��=Cu2����H2O��Fe��2Fe3��=3Fe2����Fe��Cu2��=Cu��Fe2����Fe��2H��=Fe2����H2�����������������Cu����4��ʵ��õ�FeSO4��7H2O�������ȴ�ᾧ���õ�FeSO4��7H2O��II.��1������һ�����ʵ���Ũ�ȵ���Һ������Ҫ��������100mL������ƿ����2�����������ӷ�Ӧ��5Fe2����MnO4����8H��=5Fe3����Mn2����4H2O����˵ζ����յ�ı�־Ϊ�������һ�α�Һ����ʱ����Һ��Ϊ��ɫ���� 30s ���ֲ��� ����3���������ӷ�Ӧ����ʽ��n(FeSO4��7H2O)=5��10��10��3��0.1��100/25mol=0.02mol������������Ϊ0.02��278/10��100%=55.6%����4�����ƫС��˵�����ĵĸ�����ص������С�����ݵζ��̶ܿȴ��ϵ��¿̶��������Ӧ�����ӡ�

����Ŀ��ʵ���ҳ���ʯ��ʯ��ϡ������ȡ������̼��
̽��һ ���巢��װ�õ�ѡ��

��1��д��ͼ�д�������������ƣ�a_____________��b_______________��
��2��ʵ������ȡ������̼���壬�������ռ�װ�÷ֱ�ѡ��______ ��______ ������ĸ�������鼯���ķ�����___________________________________��
̽���� ҩƷ��ѡ��
С��������ҩƷ�������о���ʵ���¼���£�
��� | ҩ Ʒ | ʵ������ |
�� | ��״ʯ��ʯ��ϡ���� | ���������������� |
�� | ��״ʯ��ʯ��ϡ���� | �����������ʻ�������ֹͣ |
�� | ��ĩ״ʯ��ʯ��ϡ���� | �����������ʺܿ� |
��3������ʵ��٢ۣ���̽��_____________________�Բ���������̼���ʵ�Ӱ�죻
��4������ʵ��______����̽����ͬ����Բ���������̼���ʵ�Ӱ�죻
��5��С��ѡ��ڢ���ҩƷ����ȡ������̼�����鷴Ӧ�Ļ�ѧ����ʽΪ___________��
̽���� ���ɶ�����̼���IJⶨ
ʵ���ҳ�ͨ����������;���������ɶ�����̼����
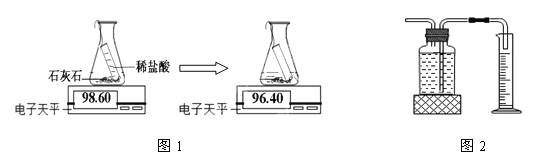
��6��;��һ����ͼ1������ͼ��֪����������̼������Ϊ________g��
;��������ͼ2����ͨ����ˮ��������ɶ�����̼�������
��7����������;���Ƚϣ�����Ϊ����;��ʵ������Ϊȷ��������___________________��
����Ŀ��������X����Y��Һ�У����ɳ������ʵ���n2������X�����ʵ���n1�Ĺ�ϵ��ͼ��ʾ������ͼʾ�������
A | B | C | D | |
X | NaOH | AlCl3 | HCl | NaAlO2 |
Y | AlCl3 | NaOH | NaAlO2 | HCl |

A. A B. B C. C D. D