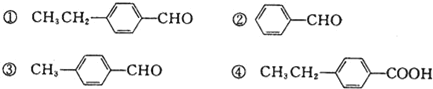
��Ŀ����
����Ŀ��(1)2 mol O3��3 mol O2������֮��Ϊ___,ͬ��ͬѹ�µ��ܶ�֮��Ϊ____,����ԭ����֮��Ϊ____��
(2)�ڱ�״����,��CO��CO2��ɵĻ������6.72 L,����Ϊ12 g.�˻������CO��CO2������Ŀ֮����________,��������ƽ��Ħ��������________,������������ܶ���________.
(3)���м��־������ĺ���������밴Ҫ������
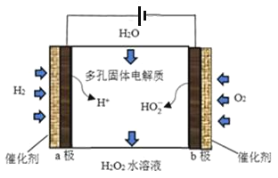
һ�������¢ٿ�ת��Ϊ�ܣ������Լ�����ʵ�ָ�ת������_________(����ĸ)��
A�����������Һ B��H2
C��������Һ/H+ D������������Һ
(4)��֪ ��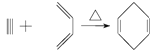 ���Ҫ�ϳ�
���Ҫ�ϳ�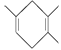 �����õ���ʼԭ�ϵ�ϵͳ����Ϊ_________________��
�����õ���ʼԭ�ϵ�ϵͳ����Ϊ_________________��
���𰸡�1:1 3:2 1:1 1:3 40 20 C 2��3-����-l,3-����ϩ�ͱ�Ȳ��2-��-l,3-����ϩ��2-��Ȳ
��������
(1)����m=nM��N=nNA����=![]() �����ÿ�������к��е�ԭ�Ӹ������������
�����ÿ�������к��е�ԭ�Ӹ������������
(2)����������n=![]() =
=![]() ���з���ʽ�����������ʵ���֮�ȣ��ٸ���M=
���з���ʽ�����������ʵ���֮�ȣ��ٸ���M=![]() ����ͬ������������ܶ�֮�ȵ�����Ħ������֮�ȷ�����
����ͬ������������ܶ�֮�ȵ�����Ħ������֮�ȷ�����
(3)һ�������¢ٿ�ת��Ϊ�ܣ�����ȩ��������������������ͭ����Һ��������Һ����������������
(4)����1��3-����ϩ����Ȳ��Ӧ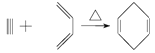 ֪��1��3-����ϩ�е�����̼̼˫�����ѣ��м��γ�һ��̼̼˫�������������������Ȳ�е���������������ɻ�״�����1��3-����ϩ����Ȳ�ļӳɷ�Ӧ��������ϳɷ�������֪��Ҫ�ϳ�
֪��1��3-����ϩ�е�����̼̼˫�����ѣ��м��γ�һ��̼̼˫�������������������Ȳ�е���������������ɻ�״�����1��3-����ϩ����Ȳ�ļӳɷ�Ӧ��������ϳɷ�������֪��Ҫ�ϳ�![]() �������ƶ�
�������ƶ�![]() ������
������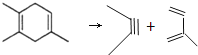 ��
��
(1)������Ħ������Ϊ48g/mol��������Ħ������Ϊ32g/mol������m=nM֪��������֮��=(48g/mol��2):(32g/mol��3)=1:1����ͬ�����£�����Ħ�������ȣ�������=![]() ֪�����ܶ�֮�ȵ�����Ħ������֮��=48g/mol:32g/mol=3:2��һ�����������к���3����ԭ�ӣ�һ�����������к���2����ԭ�ӣ��������֮��Ϊ2:3�����ÿ�������к��е���ԭ�Ӹ���֪����ԭ�Ӹ���֮��Ϊ1:1��
֪�����ܶ�֮�ȵ�����Ħ������֮��=48g/mol:32g/mol=3:2��һ�����������к���3����ԭ�ӣ�һ�����������к���2����ԭ�ӣ��������֮��Ϊ2:3�����ÿ�������к��е���ԭ�Ӹ���֪����ԭ�Ӹ���֮��Ϊ1:1��
(2)��COΪxmol��CO2Ϊymol����x+y=![]() ��28x+44y=12����ã�x=0.075��y=0.225���˻������CO��CO2�ķ�����Ŀ����0.075mol:0.225mol=1:3����������ƽ��Ħ������Ϊ
��28x+44y=12����ã�x=0.075��y=0.225���˻������CO��CO2�ķ�����Ŀ����0.075mol:0.225mol=1:3����������ƽ��Ħ������Ϊ ==40g/mol����ͬ������������ܶ�֮�ȵ�����Ħ������֮�ȣ����Ըû��������ܶ�����������ܶ���20��
==40g/mol����ͬ������������ܶ�֮�ȵ�����Ħ������֮�ȣ����Ըû��������ܶ�����������ܶ���20��
(3)һ�������¢ٿ�ת��Ϊ�ܣ�����ȩ��������������������ͭ����Һ��������Һ�������������������и�����ؾ���ǿ�����ԣ�����������������ֻ��C���ϣ��ʴ�ΪC��
(4)����1��3-����ϩ����Ȳ��Ӧ![]() ֪��1��3-����ϩ�е�����̼̼˫�����ѣ��м��γ�һ��̼̼˫�������������������Ȳ�е���������������ɻ�״�����1��3-����ϩ����Ȳ�ļӳɷ�Ӧ��������ϳɷ�������֪��Ҫ�ϳ�
֪��1��3-����ϩ�е�����̼̼˫�����ѣ��м��γ�һ��̼̼˫�������������������Ȳ�е���������������ɻ�״�����1��3-����ϩ����Ȳ�ļӳɷ�Ӧ��������ϳɷ�������֪��Ҫ�ϳ�![]() �������ƶ�
�������ƶ�![]() ������
������ ����Ϊ
����Ϊ![]() �������л��������ԭ��������ԭ�Ϸֱ���2��3-����-l��3-����ϩ�ͱ�Ȳ����Ϊ
�������л��������ԭ��������ԭ�Ϸֱ���2��3-����-l��3-����ϩ�ͱ�Ȳ����Ϊ �����л��������ԭ��������ԭ�Ϸֱ���2-��-l,3-����ϩ��2-��Ȳ��
�����л��������ԭ��������ԭ�Ϸֱ���2-��-l,3-����ϩ��2-��Ȳ��

 ���ɶ��ȫ���ƿؾ�ϵ�д�
���ɶ��ȫ���ƿؾ�ϵ�д�����Ŀ�������±���Ϣ(���ֶ�����Ԫ�ص�ԭ�Ӱ뾶����Ҫ���ϼ�)��֪������������ȷ����
Ԫ�ش��� | A | B | C | D | E |
ԭ�Ӱ뾶/nm | 0.186 | 0.143 | 0.089 | 0.104 | 0.074 |
��Ҫ���ϼ� | +1 | +3 | +2 | +6��-2 | -2 |
A.���Ӱ뾶��С��![]()
B.![]() ��
��![]() �����������������
�����������������
C.����������Ӧˮ����ļ��ԣ�A<C
D.�⻯����ȶ��ԣ�D>E
����Ŀ�����б�Ŵ���Ԫ�����ڱ��е�һ����Ԫ�أ��û�ѧʽ�ش��������⣺
| ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
�� | �� | �� | �� | |||||
�� | �� | �� | �� | �� | �� | |||
�� | �� | �� |
(1)�١��ۡ��ݵ�����������ˮ���������ǿ����˳��Ϊ___________(�ѧʽ����ͬ)��
(2)�ڡ��ۡ����γɵļ������Ӱ뾶�ɴ�С��˳����______________��
(3)��͢������������Ӧ��ˮ���ﻯѧʽΪ_______��_________�����������е�ȼ���ɵ���ɫ�Ĺ��壬�ù���ĵ���ʽΪ_______________�����ܹ���ˮ���ҷ�Ӧ����д���÷�Ӧ�Ļ�ѧ����ʽ_______________����Ԫ�آ�ĵ���ͨ��NaBr��Һ�У���Ӧ�����ӷ���ʽΪ_______________��
(4)�ޡ��ߡ�������Ԫ���γɵ���̬�⻯����ȶ���������____________(�ѧʽ����ͬ)������Ԫ�طǽ�������ǿ������˳��Ϊ___________��