��Ŀ����
����Ŀ��������������: ͬ���칹�����д
��1��ij��������ʽΪC7H16 ��д��������������������ͬ���칹��Ľṹ��ʽ�� ____________________________________________
��2��1 mol�л�����1 mol H2�����ӳɷ�Ӧ����ӳɺ�IJ�����2,2,3���������飬����л�����ܵĽṹ��ʽ��__________��__________��__________��
��3��1molij������1mol�Ȼ�����ȫ�ӳɣ��ӳɲ�������ϵ���ԭ���ֿɱ�9mol����ȡ���������ķ���ʽΪ_____________����д�������п����Ľṹ��ʽ________________________________________________________________
ѧ���⣺ͨ������Ľ���ܽ�ͬ���칹�峣��������
���𰸡� 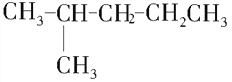 ��
�� 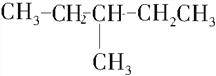
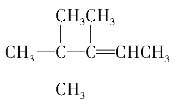
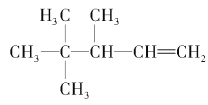
 C4H8 ����ͬ���칹�ṹ��ʽ��
C4H8 ����ͬ���칹�ṹ��ʽ��
����������1�������ṹʽ�к���3�������������ֻ��1��֧������֧��Ϊ-CH3����������6��̼ԭ�ӣ����������У�CH3CH��CH3��CH2CH2CH2CH3��CH3CH2CH��CH3��CH2CH2CH3����֧��Ϊ-CH2CH3����������5��̼ԭ�ӣ����������У�CH3CH2CH��CH2CH3��CH2CH3��
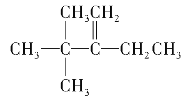
��2������ϩ����H2�ӳɷ�Ӧ��ԭ������֪����������������̼ԭ���Ͼ�����ԭ�ӵ�̼ԭ�Ӽ��Ƕ�Ӧϩ������̼̼˫����λ�ã���������̼���ṹΪ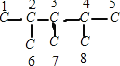 ��1�ź�5��̼ԭ�ӹ���3��̼ԭ�ӶԳƣ��������γ�˫��λ���У�1��2֮�䣨��4��5������2��6֮�䣩����4��8������2��3֮�䣨��4��3����3��7֮�䣬�ʸ�������3�֣������л�����ܵĽṹ��ʽΪCH2=C��CH3��CH��CH3��CH��CH3��2��CH3C��CH3��=C��CH3��CH��CH3��2��[��CH3��2CH]2C=CH2��
��1�ź�5��̼ԭ�ӹ���3��̼ԭ�ӶԳƣ��������γ�˫��λ���У�1��2֮�䣨��4��5������2��6֮�䣩����4��8������2��3֮�䣨��4��3����3��7֮�䣬�ʸ�������3�֣������л�����ܵĽṹ��ʽΪCH2=C��CH3��CH��CH3��CH��CH3��2��CH3C��CH3��=C��CH3��CH��CH3��2��[��CH3��2CH]2C=CH2��
��3��1molij������1mol�Ȼ�����ȫ�ӳɣ�˵������Ϊ��ϩ���������Ļ�ѧʽΪCnH2n���ӳɲ���ΪCnH2n+1Cl��������ϵ���ԭ���ֿɱ�9mol����ȡ������2n+1=9��n=4�������ķ���ʽΪC4H8����д�������п��ܵĽṹ��ʽ��CH2=CHCH2CH3��CH3CH=CHCH3����CH2=C(CH3)CH2����

