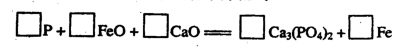��Ŀ����
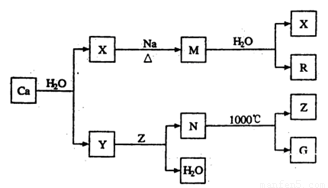
��11�֣������������������ʸֲĵ�����������ij���ߵ���Ҫ�ɷ�Ϊ����M��Ca��
��1��Ca��ԭ�ӽṹʾ��ͼ ��
��2����ƽ�ø��������۵Ļ�ѧ����ʽ��
P + FeO + CaO
 Ca3(PO4)2
+ Fe
Ca3(PO4)2
+ Fe
��3����������������ϡ����������NaOH��Һ�����ɰ�ɫ��״������Ѹ�ٱ�ɻ���ɫ������ɺ��ɫM(OH)n�������MΪ_________�����Mn+ �ķ�����_____________________�������ӷ���ʽ�����
��4��ȡ1.6g������������ˮ��ַ�Ӧ������224ml H2����״������������Һ��ͨ��������CO2������ܵõ�CaCO3 g��
��5���������ֳ���CO��SO2�̵�����Ⱦ��һ�ַ������ǽ����ڴ���������ת��Ϊ����S��
��֪��CO(g)
+ 1/2 O2(g) == CO2(g)
 H = ��283.0 kJ��mol��2
H = ��283.0 kJ��mol��2
S(s) + O2(g) == YO2(g)
 H = ��296.0 kJ��mol��1
H = ��296.0 kJ��mol��1
�˷�Ӧ���Ȼ�ѧ����ʽ������������������������������������������������������
����11�֣�
��1��  ��2�֣�
��2�֣�
��2��2P + 5FeO +
3CaO  Ca3(PO4)2 +
5Fe ��2�֣�
Ca3(PO4)2 +
5Fe ��2�֣�
��3��Fe ��1�֣� Fe3+ + 3SCN�� == Fe(SCN)3 ��2�֣�
��4��1.0 ��2�֣�
��5��2CO(g) + SO2(g) == S(s)
+ 2CO2 (g)  H = -270kJ/mol ��2�֣�
H = -270kJ/mol ��2�֣�
����������

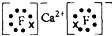
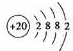
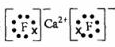
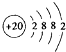
 Ԫ�������ڱ���λ���� ����ԭ�ӽṹʾ��ͼ ��
Ԫ�������ڱ���λ���� ����ԭ�ӽṹʾ��ͼ ��