��Ŀ����
����Ŀ��ijͬѧ������80mL2.0mol/LNaOH��Һ����ת����Һ�IJ�����ͼ��ʾ��
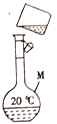
��1��ͼ�в����ϵĴ�����________��
��2��ͼ���õ�������M��_______(���������ƺ��)�����й�������M��ʹ�÷����У���ȷ����_______ (����ĸ)��
a.ʹ��ǰӦ����Ƿ�©Һ b.ʹ��ǰ������
c.�����������ʷ�Ӧ���ܽ������ d.��ֱ�ӽ�����Һת�Ƶ�����
��3����ͬѧ��ȡNaOH���������Ϊ________��
��4�����в�����ʹ���Ƶ���ҺŨ��ƫ�ߵ���______(����ĸ)��
a.���ù����ձ�δϴ��
b.ת�ƹ�������������Һ����
c.ҡ�Ⱥ�,Һ���½����ټ���ˮ���̶���
d.����ʱ���ӿ̶���
���𰸡� δ�ò��������� 100mL����ƿ ac 8.0g d
����������1��ͼ�в���������һ�����ʵ���Ũ�ȵ���Һʱת����Һ�����ֵĴ�����δ�ò�������������2��80 mL��������ƿ��Ҫ����80mL2.0mol/LNaOH��Һ������ѡ��100mL����ƿ����ͼ���õ�������M��100mL����ƿ������ƿ��ʹ��ǰ��Ҫ����Ƿ�©Һ��ѡ��a��ȷ�����������ʵ���Ũ����Һʱû�б�Ҫ��ɣ�ѡ��b��������ƿ�����������ʷ�Ӧ���ܽ��������ѡ��c��ȷ����ת����Һ֮ǰҪ��ȴ������ֱ�ӽ�����Һת�Ƶ����У�ѡ��d����ѡac����3����ȡNaOH���������Ϊ�� 2.0mol/L��0.1L��40g/mol=8.0g����4��a. �ܽ�ʱ��Ҫ���������ù����ձ�δϴ�ӣ���������Һ��Ũ����Ӱ�죻b.ת�ƹ�������������Һ���������ʼ��٣����ݺ���Һ��Ũ��ƫ�ͣ�c.ҡ�Ⱥ���Һ���½����ټ���ˮ���̶��ߣ��൱��ˮ�Ӷ��ˣ���Һ��Ũ��ƫ�ͣ�d.����ʱ���ӿ̶��ߣ����ӵ�����ˮ���ˣ���Һ��Ũ��ƫ�ߣ���ѡd��