��Ŀ����
��8�֣�������������Ҫ���л��ϳ��м��壬�㷺Ӧ���ڻ�ѧ��ҵ��ʵ����������ͼ��װ���Ʊ�����������
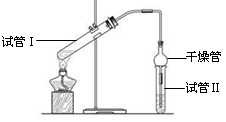
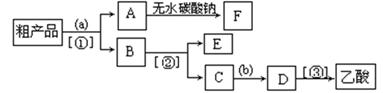
��1����̲IJ��õ�ʵ��װ�ò�ͬ����װ���в��������θ���ܣ��������ǣ� ��
��2��Ϊ��֤��Ũ�����ڸ÷�Ӧ�����˴�������ˮ�������ã�ijͬѧ������ͼ��ʾװ�ý���������4��ʵ�顣ʵ�鿪ʼ���þƾ�����3min���ټ���ʹ֮����3min��ʵ�����������С�Թܢ��ٲ��л���ĺ�ȣ�ʵ���¼���£�
��ʵ��D��Ŀ������ʵ��C����գ�֤��H+��������Ӧ���д����á�ʵ��D��Ӧ��������������Ũ�ȷֱ���______mL��_____mol��L��1��
�ڷ���ʵ�� ����ʵ���ţ������ݣ������Ʋ��ŨH2SO4����ˮ����������������IJ��ʡ�
��3������������90g���Ҵ�138g����������Ӧ�õ�80g�����������Լ���÷�Ӧ�IJ���Ϊ______________��

��1����̲IJ��õ�ʵ��װ�ò�ͬ����װ���в��������θ���ܣ��������ǣ� ��
��2��Ϊ��֤��Ũ�����ڸ÷�Ӧ�����˴�������ˮ�������ã�ijͬѧ������ͼ��ʾװ�ý���������4��ʵ�顣ʵ�鿪ʼ���þƾ�����3min���ټ���ʹ֮����3min��ʵ�����������С�Թܢ��ٲ��л���ĺ�ȣ�ʵ���¼���£�
| ʵ���� | �Թܢ����Լ� | �Թܢ��� �Լ� | �л���� ���/cm |
| A | 2 mL�Ҵ���1 mL���ᡢ 1mL18mol��L��1Ũ���� | ����Na2CO3 ��Һ | 3.0 |
| B | 2 mL�Ҵ���1 mL���� | 0.1 | |
| C | 2 mL�Ҵ���1 mL���ᡢ 3 mL 2mol��L��1 H2SO4 | 0.6 | |
| D | 2 mL�Ҵ���1 mL���ᡢ���� | 0.6 |
�ڷ���ʵ�� ����ʵ���ţ������ݣ������Ʋ��ŨH2SO4����ˮ����������������IJ��ʡ�
��3������������90g���Ҵ�138g����������Ӧ�õ�80g�����������Լ���÷�Ӧ�IJ���Ϊ______________��
(��8��)��1�������ͷ�ֹ������2�֣���
��2����3 4 ����1�֣� ��A C��2��,��ѡ���÷֣�©ѡ��1�֣� ��3�� 60.6%
��2����3 4 ����1�֣� ��A C��2��,��ѡ���÷֣�©ѡ��1�֣� ��3�� 60.6%
��1��Ӧ��������Ҵ����Ǻ�ˮ���ܵģ����Կ����ֹ���������ã�ͬʱҲ���������á�
��2���ټ�Ȼ�Ƕ���ʵ�飬�������ӵ�Ũ��Ӧ����ͬ�ģ����������Ũ����4mol/L�������3ml��
�ڸ���A��C�����ɵ������������������жϣ�ŨH2SO4����ˮ����������������IJ��ʡ�
��3�����ݷ�ӦʽCH3COOH��CH3CH2OH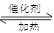 CH3COOCH2CH3��H2O��֪��90g����Ӧ�������Ҵ�69g������������Ӧ����������������132g���Ҵ�������80��132��100����60.6����
CH3COOCH2CH3��H2O��֪��90g����Ӧ�������Ҵ�69g������������Ӧ����������������132g���Ҵ�������80��132��100����60.6����
��2���ټ�Ȼ�Ƕ���ʵ�飬�������ӵ�Ũ��Ӧ����ͬ�ģ����������Ũ����4mol/L�������3ml��
�ڸ���A��C�����ɵ������������������жϣ�ŨH2SO4����ˮ����������������IJ��ʡ�
��3�����ݷ�ӦʽCH3COOH��CH3CH2OH
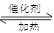 CH3COOCH2CH3��H2O��֪��90g����Ӧ�������Ҵ�69g������������Ӧ����������������132g���Ҵ�������80��132��100����60.6����
CH3COOCH2CH3��H2O��֪��90g����Ӧ�������Ҵ�69g������������Ӧ����������������132g���Ҵ�������80��132��100����60.6����
��ϰ��ϵ�д�
�����Ŀ
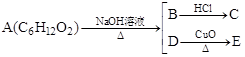
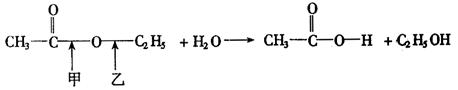



 ��
��