��Ŀ����
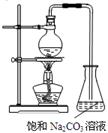
��14�֣�ʵ���Һϳ����������IJ������£���Բ����ƿ�ڼ����Ҵ���Ũ��������ᣬƿ����ֱ��װͨ����ȴˮ�������ܣ�ʹ��Ӧ��������������ΪҺ��������ƿ�ڣ������Ȼ���һ��ʱ�������װ�ý�����������ͼ��ʾ�����õ������Ҵ��������ˮ�����������ֲ�Ʒ����ش��������⣺
����֪���Ҵ������ᡢ���������ķе�������78.4�桢118�桢77.1�棩
��1������ƿ�г��˼����Ҵ���Ũ����������⣬��Ӧ���뼸�����Ƭ��
��Ŀ����_________________________��
��2������ƿ�м���һ���������Ҵ���Ũ����Ļ��Һ�ķ����� ��
��3���������������ķ�Ӧ�ǿ��淴Ӧ����Ӧ�ﲻ����ȫת��Ϊ�������Ӧһ��ʱ��ʹﵽ�˸÷�Ӧ���ȣ����ﵽ��ѧƽ��״̬������������˵���÷�Ӧ�Ѵﵽ��ѧƽ��״̬����(�����)������������������
�ٵ�λʱ�������1mol����������ͬʱ����1molˮ
�ڵ�λʱ�������1mol����������ͬʱ����1mol����
�۵�λʱ�������1mol�Ҵ���ͬʱ����1mol����
������Ӧ���������淴Ӧ��������� �ݻ�����и����ʵ�Ũ�Ȳ��ٱ仯
��4����ÿ��1�֣�������뺬���ᡢ�Ҵ���ˮ�����������ֲ�Ʒ����ͼ�Ƿ��������������ͼ������ͼ��Բ�����������ʵ����Լ����ڷ������������ʵ��ķ��뷽����
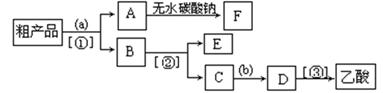
EΪ____________(������)���Լ�a��__________���Լ�bΪ_______�����뷽������___________�����뷽������__________________�����뷽������__________��
��5��д��C �� D ��Ӧ�Ļ�ѧ����ʽ ��
����֪���Ҵ������ᡢ���������ķе�������78.4�桢118�桢77.1�棩
��1������ƿ�г��˼����Ҵ���Ũ����������⣬��Ӧ���뼸�����Ƭ��
��Ŀ����_________________________��
��2������ƿ�м���һ���������Ҵ���Ũ����Ļ��Һ�ķ����� ��
��3���������������ķ�Ӧ�ǿ��淴Ӧ����Ӧ�ﲻ����ȫת��Ϊ�������Ӧһ��ʱ��ʹﵽ�˸÷�Ӧ���ȣ����ﵽ��ѧƽ��״̬������������˵���÷�Ӧ�Ѵﵽ��ѧƽ��״̬����(�����)������������������
�ٵ�λʱ�������1mol����������ͬʱ����1molˮ
�ڵ�λʱ�������1mol����������ͬʱ����1mol����
�۵�λʱ�������1mol�Ҵ���ͬʱ����1mol����
������Ӧ���������淴Ӧ��������� �ݻ�����и����ʵ�Ũ�Ȳ��ٱ仯
��4����ÿ��1�֣�������뺬���ᡢ�Ҵ���ˮ�����������ֲ�Ʒ����ͼ�Ƿ��������������ͼ������ͼ��Բ�����������ʵ����Լ����ڷ������������ʵ��ķ��뷽����

EΪ____________(������)���Լ�a��__________���Լ�bΪ_______�����뷽������___________�����뷽������__________________�����뷽������__________��
��5��д��C �� D ��Ӧ�Ļ�ѧ����ʽ ��
��1����ֹ��ƿ��Һ�屩��
��2��������ƿ�м���һ�������Ҵ���Ȼ��������Ũ���������ƿ���ӱ���
��3���ڢܢ�
��4����ÿ��1�֣��Ҵ� ����̼������Һ Ũ���� ��Һ ���� ����
��5��2CH3COONa + H2SO4 = Na2SO4 + 2CH3COOH
��2��������ƿ�м���һ�������Ҵ���Ȼ��������Ũ���������ƿ���ӱ���
��3���ڢܢ�
��4����ÿ��1�֣��Ҵ� ����̼������Һ Ũ���� ��Һ ���� ����
��5��2CH3COONa + H2SO4 = Na2SO4 + 2CH3COOH
��1��������Ӧ��Ҫ���ȣ�Ϊ�˷�ֹҺ�����ʱ������������Ҫ���Ƭ��ֹ���С�
��2��Ũ��������ˮ�ų��������ȣ���Ũ������ܶȴ���ˮ�ġ�Ϊ�˷�ֹ�Ҵ�������Ļӷ�����Ҫ��Ũ����ע���Ҵ��У�����ȴ���ټ������ᡣ
��3���ٺ͢��з�Ӧ���ʵķ�������ͬ�����κ�����¶�����������ȷ�����з�Ӧ���ʵķ����෴��������֮��������Ӧ�Ļ�ѧ������֮�ȣ���ȷ���ܢݾ����ϻ�ѧƽ����ص㣬����ȷ�ġ���ѡ�ڢܢ�
��4���ֲ�Ʒ�к����Ҵ������ᣬ��Ҫ���ñ���̼������Һ��ȥ������Ҵ���Ȼ���Һ���õ�����������ҺA�����ͨ����ˮ̼���Ƶõ���������F��B�к����Ҵ����������Լ�ʣ���̼���ơ����Կ�ֱ������õ��Ҵ�����ʣ�����Һ�м���Ũ���ἴ�õ����ᣬ�����ͨ������õ����ᡣ
��5�������ƺ����ᷴӦ���ɴ���������ơ�
��2��Ũ��������ˮ�ų��������ȣ���Ũ������ܶȴ���ˮ�ġ�Ϊ�˷�ֹ�Ҵ�������Ļӷ�����Ҫ��Ũ����ע���Ҵ��У�����ȴ���ټ������ᡣ
��3���ٺ͢��з�Ӧ���ʵķ�������ͬ�����κ�����¶�����������ȷ�����з�Ӧ���ʵķ����෴��������֮��������Ӧ�Ļ�ѧ������֮�ȣ���ȷ���ܢݾ����ϻ�ѧƽ����ص㣬����ȷ�ġ���ѡ�ڢܢ�
��4���ֲ�Ʒ�к����Ҵ������ᣬ��Ҫ���ñ���̼������Һ��ȥ������Ҵ���Ȼ���Һ���õ�����������ҺA�����ͨ����ˮ̼���Ƶõ���������F��B�к����Ҵ����������Լ�ʣ���̼���ơ����Կ�ֱ������õ��Ҵ�����ʣ�����Һ�м���Ũ���ἴ�õ����ᣬ�����ͨ������õ����ᡣ
��5�������ƺ����ᷴӦ���ɴ���������ơ�

��ϰ��ϵ�д�
�����Ŀ
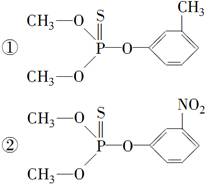


 ֪���ٵ����������м�ʱ�������������������Ҫ���������λ���λ���������������Ȼ�ʱ�������������������Ҫ�����Ȼ��ļ�λ��
֪���ٵ����������м�ʱ�������������������Ҫ���������λ���λ���������������Ȼ�ʱ�������������������Ҫ�����Ȼ��ļ�λ��
 �к��еĺ��������ŵ�����Ϊ���� ��
�к��еĺ��������ŵ�����Ϊ���� �� ��
�� ��
�� �нϺõ���ȼ�ԣ���д���Լױ�Ϊ��Ҫԭ���Ʊ�����ȼ���ĺϳ�·������ͼ��
�нϺõ���ȼ�ԣ���д���Լױ�Ϊ��Ҫԭ���Ʊ�����ȼ���ĺϳ�·������ͼ��
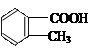 ���ж���ͬ���칹�壬�����������࣬�ҷ��ӽṹ
���ж���ͬ���칹�壬�����������࣬�ҷ��ӽṹ