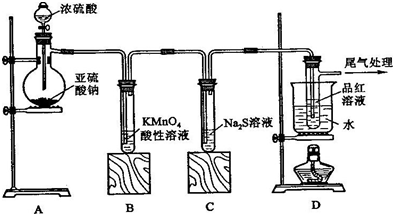
��Ŀ����
���ʵ����ǻ�ѧ�г��õ�����������������ѧ����֪ʶ������գ�
��1��SO2��Ħ��������______�� 0.1molCO2��������______g�����ڱ�״�������ԼΪ______L����0.5L 0.2mol/L�� Ba��NO3��2��Һ��NO3-�����ʵ���Ũ��Ϊ______��
��2����һ���¶Ⱥ�ѹǿ�£�5�������A2��15���������B2��ȫ��������10���ij����C���������C�Ļ�ѧʽΪ����A��B��ʾ��______��
��3����״����4.48Lij���������Ϊ14.2g���������Ħ������Ϊ______��
��4�������10mol?L-1 ��Ũ��������250mL 1mol?L-1 ��ϡ���ᣬ��Ҫ��ȡ______mLŨ���ᣮ
�⣺��1��SO2��Ħ��������64g/mol�� 0.1molCO2������=0.1mol��44g/mol=4.4g�����ڱ�״�������ԼΪ0.1mol��22.4L/mol=2.24L����0.5L 0.2mol/L�� Ba��NO3��2��Һ��NO3-�����ʵ���Ũ��=0.2mol/L��2=0.4mol/L��
�ʴ�Ϊ��64g/mol��4.4��2.24��0.4mol/L��
��2����ͬ�����£����֮�ȵ������ʵ���֮�ȵ��ڷ�����֮�ȣ�����5�������A2��15�������B2��������10���ij����C������A2��B2��C�Ļ�ѧ������֮��Ϊ5�����15�����10���=1��3��2�����Է���ʽΪA2+3B2=2C������ԭ���غ��֪C�Ļ�ѧʽΪAB3��
�ʴ�Ϊ��AB3��
��3����״����4.48L��������ʵ���=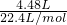 =0.2mol���������Ħ������=
=0.2mol���������Ħ������=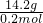 =71g/mol��
=71g/mol��
�ʴ�Ϊ��71g/mol��
��4������Ҫ10mol?L-1 ��Ũ��������ΪV������ϡ�Ͷ��ɣ���
10mol?L-1��V=250mL��1mol?L-1�����V=25.0mL��
�ʴ�Ϊ��25.0mL��
��������1��Ħ��������g/mol����λ����ֵ�ϵ�������Է�������������m=nM����0.1molCO2������������V=nVmj���������̼�������NO3-�����ʵ���Ũ��ΪBa��NO3��2Ũ�ȵ�2����
��2����ͬ�����£����֮�ȵ������ʵ���֮�ȵ��ڷ�����֮�ȣ��ݴ��жϷ�Ӧ����ʽ�����ʵĻ�ѧ�����������ݷ���ʽԭ���غ����C�Ļ�ѧʽ��
��3������n=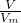 �������������ʵ������ٸ���M=
�������������ʵ������ٸ���M=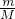 ����������Ħ��������
����������Ħ��������
��4������ϡ�Ͷ��ɼ�����Ҫ10mol?L-1 ��Ũ����������
���������鳣�û�ѧ�������йؼ��㣬�ѶȲ���ע�ʽ��������������ã�
�ʴ�Ϊ��64g/mol��4.4��2.24��0.4mol/L��
��2����ͬ�����£����֮�ȵ������ʵ���֮�ȵ��ڷ�����֮�ȣ�����5�������A2��15�������B2��������10���ij����C������A2��B2��C�Ļ�ѧ������֮��Ϊ5�����15�����10���=1��3��2�����Է���ʽΪA2+3B2=2C������ԭ���غ��֪C�Ļ�ѧʽΪAB3��
�ʴ�Ϊ��AB3��
��3����״����4.48L��������ʵ���=
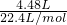 =0.2mol���������Ħ������=
=0.2mol���������Ħ������=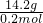 =71g/mol��
=71g/mol���ʴ�Ϊ��71g/mol��
��4������Ҫ10mol?L-1 ��Ũ��������ΪV������ϡ�Ͷ��ɣ���
10mol?L-1��V=250mL��1mol?L-1�����V=25.0mL��
�ʴ�Ϊ��25.0mL��
��������1��Ħ��������g/mol����λ����ֵ�ϵ�������Է�������������m=nM����0.1molCO2������������V=nVmj���������̼�������NO3-�����ʵ���Ũ��ΪBa��NO3��2Ũ�ȵ�2����
��2����ͬ�����£����֮�ȵ������ʵ���֮�ȵ��ڷ�����֮�ȣ��ݴ��жϷ�Ӧ����ʽ�����ʵĻ�ѧ�����������ݷ���ʽԭ���غ����C�Ļ�ѧʽ��
��3������n=
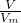 �������������ʵ������ٸ���M=
�������������ʵ������ٸ���M=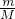 ����������Ħ��������
����������Ħ����������4������ϡ�Ͷ��ɼ�����Ҫ10mol?L-1 ��Ũ����������
���������鳣�û�ѧ�������йؼ��㣬�ѶȲ���ע�ʽ��������������ã�

��ϰ��ϵ�д�
�����Ŀ
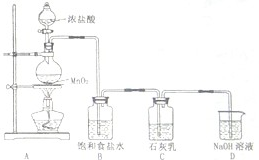 ��ˮռ��������ˮ����97.2%�����Ѻ�ˮ�����ͻ�����������������ȿ��Խ����ˮ��Դȱ�������⣬�ֿ��Գ�����ú�����Դ��
��ˮռ��������ˮ����97.2%�����Ѻ�ˮ�����ͻ�����������������ȿ��Խ����ˮ��Դȱ�������⣬�ֿ��Գ�����ú�����Դ�� ��2010?��������ģ��������һ����Ҫ�Ļ���ԭ�ϣ���ҵ�������������Ҫ�������£�
��2010?��������ģ��������һ����Ҫ�Ļ���ԭ�ϣ���ҵ�������������Ҫ�������£�