��Ŀ����
����������������Ʒ��
������̨������Ȧ����ʽ���У� ����ƿ ����ʽ�ζ��ܺͼ�ʽ�ζ���
���ձ������ɣ� �ݲ����� ��ͷ�ι� ����ƽ�������룩 ����ֽ ����Ͳ �����©����
��������ҩƷ��A.NaOH���� B.��NaOH��Һ C.δ֪Ũ������ D.����ˮ E.̼������Һ��
������������ѧ��ʵ�飬�ش��������⣺
��1������ʱ��Ӧѡ�õ�����������________�����ţ���
��2������һ�����ʵ���Ũ�ȵ�NaOH��Һʱ����ȱ�ٵ�������________��
��3��������к͵ζ�ʱ����ȱ�ٵ��Լ���______________��
��4�������к͵ζ�ʱ���������ý�Ҫʢ����Һ��ϴ�����������е�________�����ţ���
��.��ʽ�ζ��� ��.��ʽ�ζ��� ��.25 mL��Ͳ ��.��ƿ
������������Ҫ������ˡ��������ʵ���Ũ����Һ���к͵ζ��Ȼ�ѧ����ʵ������������ѡ���йص��Ͳ���Ҫ��
�𰸣���1���٢ܢݢ�� ��2������ƿ ��3��ָʾ�� ��4����

��ϰ��ϵ�д�
�����Ŀ
 ��I�����в����ᵼ��ʵ����ƫ�ߵ���
��I�����в����ᵼ��ʵ����ƫ�ߵ���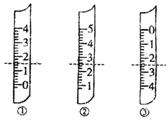 ?A������������Ϊ1.5mL
?A������������Ϊ1.5mL
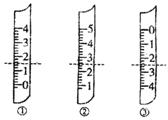 ?A������������Ϊ1.5mL
?A������������Ϊ1.5mL