��Ŀ����
 ��I�����в����ᵼ��ʵ����ƫ�ߵ���
��I�����в����ᵼ��ʵ����ƫ�ߵ���D
D
��A������һ�����ʵ���Ũ�ȵ�������Һʱ������ҡ�Ⱥ���Һ����ڿ̶��ߣ�
B��������һ�����ʵ���Ũ����Һʱ����10ml����Ͳ��ȡ5.0mlҺ������ʱ���Ӷ���
C������ƽ����20.5gij���ʣ������ҩƷ��λ�÷ŷ�������ҩƷ������
D��10%�������90%�����������������50%��������Һ
E������һ�����ʵ���Ũ����Һʱ������ʱ���Ӷ�����������Һ��Ũ��
��������������������Ʒ��
a������̨������Ȧ�����У���b����ƿ��c���ζ��ܣ�d���ձ������ɣ���e����������f����ͷ�ιܣ�g��������ƽ�������룩��h����ֽ��i����Ͳ��j��©����k���¶ȼƣ�
����գ�
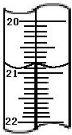
��1����ͼ��ʾ�ֱ����¶ȼơ���Ͳ���ζ��ܵ�һ���֣������жϼ����������߿̶ȣ�����ȷ����
AD
AD
��A��������Ͳ������Ϊ2.5mL
B��������Ͳ������Ϊ2.5mL
C�����ǵζ��ܣ�����Ϊ2.50mL
D�������¶ȼƣ�������2.5��
��2���ø�����������NaOH��������һ�����ʵ���Ũ�ȵ�����������Һʱ����ȱ�ٵ�������
Կ�ס�����ƿ
Կ�ס�����ƿ
����3���ø������������д��ε��ᴿʵ�飬��ȱ�ٵ�������
�����ƾ���
�����ƾ���
������������A��������Һ�ڿ̶����Ϸ�����Ӱ��������Һ�Ľ����
B�����Ӷ�����������ȡ��Һ�����ƫС��������Һ��Ũ��ƫ�ͣ�
C����Ʒ������ߵ���������������С��
D���ܶȴ�ˮ�����ʵ������Ϻ������������ڶ�������������ƽ��ֵ��
E�����Ӷ������������Ƶ���Һ�����ƫ��
����1��������Ͳ���ζ��ܡ��¶ȼƵĹ��켰ȷ�Ƚ����жϣ�
��2����������һ�����ʵ�����Ũ���õ������������жϣ�
��3�����ݴ����ᴿ�õ����������з�����
B�����Ӷ�����������ȡ��Һ�����ƫС��������Һ��Ũ��ƫ�ͣ�
C����Ʒ������ߵ���������������С��
D���ܶȴ�ˮ�����ʵ������Ϻ������������ڶ�������������ƽ��ֵ��
E�����Ӷ������������Ƶ���Һ�����ƫ��
����1��������Ͳ���ζ��ܡ��¶ȼƵĹ��켰ȷ�Ƚ����жϣ�
��2����������һ�����ʵ�����Ũ���õ������������жϣ�
��3�����ݴ����ᴿ�õ����������з�����
����⣺����A������һ�����ʵ���Ũ�ȵ�������Һʱ������ҡ�Ⱥ���Һ����ڿ̶��ߣ����������������Ӱ�����ƽ������A����
B��������һ�����ʵ���Ũ����Һʱ����10ml����Ͳ��ȡ5.0mlҺ������ʱ���Ӷ��������Ӷ�����������ȡ��Һ�������С�����Ƶ���ҺŨ��ƫС����B����
C������ƽ����20.5gij���ʣ������ҩƷ��λ�÷ŷ�������ҩƷ��������ҩƷ������ߵ���������ʵ������ƫС�����Ƶ���ҺŨ��ƫС����C����
D��10%�������90%�����������������50%��������Һ������Ũ��С��ˮ����Һ�������ϣ���Ϻ���������С��ƽ��ֵ�������ܶȴ���ˮ����Һ�������ϣ���Ϻ����Һ��������������ƽ��ֵ����D��Ϻ�������������50%����D��ȷ��
E������һ�����ʵ���Ũ����Һʱ������ʱ���Ӷ��������Ӷ������������Ƶ���Һ�����ƫ�����Ƶ���Һ��Ũ��ƫС����E����
��ѡD��
����1��A������Ͳû��0�̶ȣ�ͼ����̶������п̶ȣ�Ӧ�����¶ȼƣ���A��ȷ��
B����û����̶ȣ�����Ͳ��������2.5mL����B����
C������̶����Ϸ����ǵζ��ܣ�������2.50mL����C����
D��������Ͳ�������¶ȼƣ���D��ȷ��
��ѡAD��
��2����ȱ��ȡ�������ƹ����Կ��������Һ������ƿ��
�ʴ�Ϊ��ҩ�ס�����ƿ��
��3�������ᴿ��Ҫ���Ⱥ������������õ�������;ƾ��ƣ�
�ʴ�Ϊ�������ƾ��ƣ�
B��������һ�����ʵ���Ũ����Һʱ����10ml����Ͳ��ȡ5.0mlҺ������ʱ���Ӷ��������Ӷ�����������ȡ��Һ�������С�����Ƶ���ҺŨ��ƫС����B����
C������ƽ����20.5gij���ʣ������ҩƷ��λ�÷ŷ�������ҩƷ��������ҩƷ������ߵ���������ʵ������ƫС�����Ƶ���ҺŨ��ƫС����C����
D��10%�������90%�����������������50%��������Һ������Ũ��С��ˮ����Һ�������ϣ���Ϻ���������С��ƽ��ֵ�������ܶȴ���ˮ����Һ�������ϣ���Ϻ����Һ��������������ƽ��ֵ����D��Ϻ�������������50%����D��ȷ��
E������һ�����ʵ���Ũ����Һʱ������ʱ���Ӷ��������Ӷ������������Ƶ���Һ�����ƫ�����Ƶ���Һ��Ũ��ƫС����E����
��ѡD��
����1��A������Ͳû��0�̶ȣ�ͼ����̶������п̶ȣ�Ӧ�����¶ȼƣ���A��ȷ��
B����û����̶ȣ�����Ͳ��������2.5mL����B����
C������̶����Ϸ����ǵζ��ܣ�������2.50mL����C����
D��������Ͳ�������¶ȼƣ���D��ȷ��
��ѡAD��
��2����ȱ��ȡ�������ƹ����Կ��������Һ������ƿ��
�ʴ�Ϊ��ҩ�ס�����ƿ��
��3�������ᴿ��Ҫ���Ⱥ������������õ�������;ƾ��ƣ�
�ʴ�Ϊ�������ƾ��ƣ�
���������⿼�����к͵ζ���������������ѡ�õ�֪ʶ��ע�ؿ����˻���֪ʶ�ͻ��������������Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
 �ӵ�ʳ������ʳ�������ӵ���أ���ʳ�κ���������I���㣩����20��50mg/kg�������������Σ�������Լ��ʳ�κ������DZ��ϼӵ�ʳ��������һ����Ҫ������ijѧ����ʵ���Ҳⶨʳ�þ������Ƿ⼰�ⶨ����������Ҫ��ӳΪ��
�ӵ�ʳ������ʳ�������ӵ���أ���ʳ�κ���������I���㣩����20��50mg/kg�������������Σ�������Լ��ʳ�κ������DZ��ϼӵ�ʳ��������һ����Ҫ������ijѧ����ʵ���Ҳⶨʳ�þ������Ƿ⼰�ⶨ����������Ҫ��ӳΪ��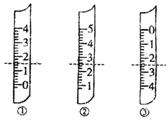 ?A������������Ϊ1.5mL
?A������������Ϊ1.5mL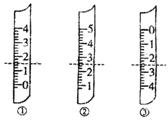 ?A������������Ϊ1.5mL
?A������������Ϊ1.5mL