��Ŀ����
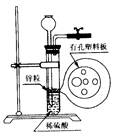
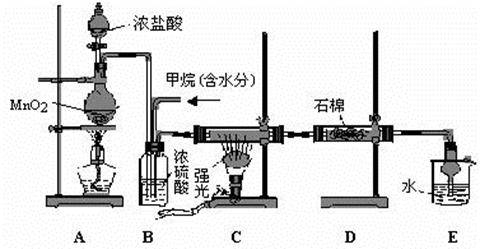
��11�֣�ʹ�����շ�������ȡ���壬������ʱ�ɿ��Ʒ�Ӧ���к�ֹͣ���ŵ㡣ij��ѧ��ȤС����������ͼ��ʾ��װ��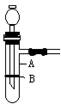 ���������շ�������
���������շ�������
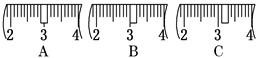
��1��ͼ��A������������ ��ȱ�ٵ������� ��
��2������Bʹ�õ��ǣ� ��
A����Ƭ B������ C��ֽƬ D������Ƭ
��3���ڸ�װ���������з�Ӧ�����壬��Ӧԭ�����У��ҿ��ܴﵽ�����ŵ���ǣ� ��
A��Na2CO3��10H2O+HCl��CO2�� B��Cu+H2SO4��H2�� C��CaCO3+HNO3��CO2��
��4�����ø�װ�ý���п����ϡ���ᷴӦ��ȡ������ʵ�飻��װ���������Լ��ķ����� ��
��5����װ���м���п���ķ����� ��
��6�����跴Ӧֹͣʱ�IJ����ͷ����������� ��
 ���������շ�������
���������շ���������1��ͼ��A������������ ��ȱ�ٵ������� ��
��2������Bʹ�õ��ǣ� ��
A����Ƭ B������ C��ֽƬ D������Ƭ
��3���ڸ�װ���������з�Ӧ�����壬��Ӧԭ�����У��ҿ��ܴﵽ�����ŵ���ǣ� ��
A��Na2CO3��10H2O+HCl��CO2�� B��Cu+H2SO4��H2�� C��CaCO3+HNO3��CO2��
��4�����ø�װ�ý���п����ϡ���ᷴӦ��ȡ������ʵ�飻��װ���������Լ��ķ����� ��
��5����װ���м���п���ķ����� ��
��6�����跴Ӧֹͣʱ�IJ����ͷ����������� ��
��1������©����ֹˮ�У����ӣ����У� ��2��D����1�֣� ��3��C��2�֣�
��4���ر�ֹˮ�У�������©���м�ˮ����ûĩ�ˣ���ʹ©����Һ������Թܣ�һ��ʱ��߶Ȳ�䣬˵����©����2�֣�
��5�����Թܺ�ţ������Ӽ�ȡп�������Թܿڣ�����������2�֣�
��6���ر�ֹˮ�У��Թ���Һ���½�������©����Һ��������2�֣�
��4���ر�ֹˮ�У�������©���м�ˮ����ûĩ�ˣ���ʹ©����Һ������Թܣ�һ��ʱ��߶Ȳ�䣬˵����©����2�֣�
��5�����Թܺ�ţ������Ӽ�ȡп�������Թܿڣ�����������2�֣�
��6���ر�ֹˮ�У��Թ���Һ���½�������©����Һ��������2�֣�
��

��ϰ��ϵ�д�
 ����С��ʿ���������ϵ�д�
����С��ʿ���������ϵ�д�
�����Ŀ
 ���л��ܼ���������ˮ���ܶ�1.46��1.52g/cm3���ж�������ʱ����Fe2(CO)9��60�淢����ȼ�����������Ϳ������������ȡ����ʻ��������Ʊ�ԭ�����£�
���л��ܼ���������ˮ���ܶ�1.46��1.52g/cm3���ж�������ʱ����Fe2(CO)9��60�淢����ȼ�����������Ϳ������������ȡ����ʻ��������Ʊ�ԭ�����£�
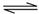 Fe(CO)5(g)��ƽ�ⳣ������ʽΪ
Fe(CO)5(g)��ƽ�ⳣ������ʽΪ
 ��Һ11.72g��ȫȼ�գ��õ�30.536gCO2��5.4gH2O��1.6g����ɫ��ĩ��
��Һ11.72g��ȫȼ�գ��õ�30.536gCO2��5.4gH2O��1.6g����ɫ��ĩ�� ��
��

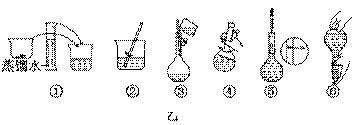
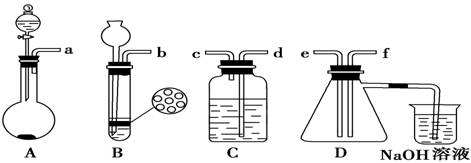
 NaOH����ʱ�����ڸ�����ѡȡ����������С��������������ĸ����������ͼ��ѡ������ȷ��ʾ����λ�õ�ѡ����������������ĸ��.
NaOH����ʱ�����ڸ�����ѡȡ����������С��������������ĸ����������ͼ��ѡ������ȷ��ʾ����λ�õ�ѡ����������������ĸ��.