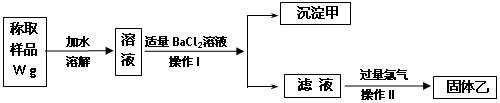
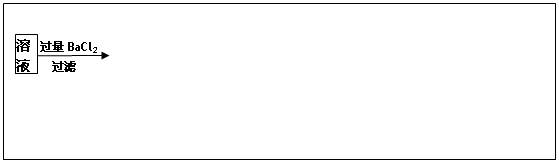
��Ŀ����
ijͬѧ�õ������壨CuSO4��5H2O ������0.40mol/L��CuSO4��Һ1000mL��
�ش��������⣺
��1����������Ϊ��(1000mL)����ƿ��������ƽ��ҩ�ס� ��ͷ�ι� ,����Ҫ��Щ��������������ɸ�ʵ�飬��д������___________ ����___________��������
��2��д����ʵ�鲽�� �ټ��� �ڳ�������__________g �� ___________ ��ת�� ��ϴ�Ӳ�ת�� ���� ��ҡ��
��3����������������Ƶ�CuSO4��ҺŨ���к�Ӱ�죿���á�ƫ��ƫС������Ӱ�족��д��
A������ƿ������ϴ�Ӻ������������ˮ________________
B�����ù����ձ���������δϴ��________________?
C������ʱ���ӿ̶���________________?
�ش��������⣺
��1����������Ϊ��(1000mL)����ƿ��������ƽ��ҩ�ס� ��ͷ�ι� ,����Ҫ��Щ��������������ɸ�ʵ�飬��д������___________ ����___________��������
��2��д����ʵ�鲽�� �ټ��� �ڳ�������__________g �� ___________ ��ת�� ��ϴ�Ӳ�ת�� ���� ��ҡ��
��3����������������Ƶ�CuSO4��ҺŨ���к�Ӱ�죿���á�ƫ��ƫС������Ӱ�족��д��
A������ƿ������ϴ�Ӻ������������ˮ________________
B�����ù����ձ���������δϴ��________________?
C������ʱ���ӿ̶���________________?
��1���ձ� ������
��2��100.0g �ܽ⣨����ȴ��
��3����Ӱ�� ƫС ƫ��
��2��100.0g �ܽ⣨����ȴ��
��3����Ӱ�� ƫС ƫ��
��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
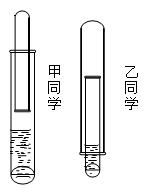
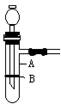 ���������շ�������
���������շ�������