��Ŀ����
�̶�������CO2������Ч��������Դ�������ٿ����е��������塣��ҵ�������о�����CO2�������״�ȼ�ϵķ������÷����Ļ�ѧ����ʽ�ǣ�
CO2��g����3H2��g�� CH3OH��g����H2O��g��
CH3OH��g����H2O��g��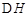 =-49��0kJ��mol
=-49��0kJ��mol
ij��ѧʵ��С�齫6mol CO2��8mol H2����һ�ݻ�Ϊ2L���ܱ������У��¶ȱ��ֲ��䣩�����H2�����ʵ�����ʱ��仯����ͼ��ʵ����ʾ��ͼ����ĸ������ֱ�ʾ��Ӧ�����꣩���ش��������⣺
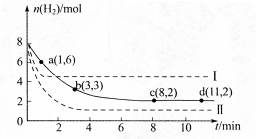
��1���÷�Ӧ��0~8min��CO2��ƽ����Ӧ������ mol��L-1��min-1
��2�����¶��¸÷�Ӧ��ƽ�ⳣ��K����ֵΪ ��
��3�����ı�ijһ�����ٽ���ʵ�飬���H2�����ʵ�����ʱ��仯��ͼ��������ʾ��
��ʵ����ȣ����ߢ�ı������������ �����ߢ�ı������������ ����ʵ�߶�Ӧ������ƽ�ⳣ��Ϊ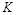 �����ߢ��Ӧ������ƽ�ⳣ��Ϊ
�����ߢ��Ӧ������ƽ�ⳣ��Ϊ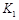 �����ߢ��Ӧ������ƽ�ⳣ��Ϊ
�����ߢ��Ӧ������ƽ�ⳣ��Ϊ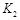 ����
����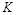 ��
��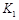 ��
��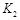 �Ĵ�С��ϵ�� ��
�Ĵ�С��ϵ�� ��
CO2��g����3H2��g��
 CH3OH��g����H2O��g��
CH3OH��g����H2O��g��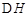 =-49��0kJ��mol
=-49��0kJ��molij��ѧʵ��С�齫6mol CO2��8mol H2����һ�ݻ�Ϊ2L���ܱ������У��¶ȱ��ֲ��䣩�����H2�����ʵ�����ʱ��仯����ͼ��ʵ����ʾ��ͼ����ĸ������ֱ�ʾ��Ӧ�����꣩���ش��������⣺

��1���÷�Ӧ��0~8min��CO2��ƽ����Ӧ������ mol��L-1��min-1
��2�����¶��¸÷�Ӧ��ƽ�ⳣ��K����ֵΪ ��
��3�����ı�ijһ�����ٽ���ʵ�飬���H2�����ʵ�����ʱ��仯��ͼ��������ʾ��
��ʵ����ȣ����ߢ�ı������������ �����ߢ�ı������������ ����ʵ�߶�Ӧ������ƽ�ⳣ��Ϊ
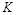 �����ߢ��Ӧ������ƽ�ⳣ��Ϊ
�����ߢ��Ӧ������ƽ�ⳣ��Ϊ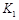 �����ߢ��Ӧ������ƽ�ⳣ��Ϊ
�����ߢ��Ӧ������ƽ�ⳣ��Ϊ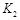 ����
����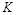 ��
��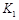 ��
��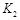 �Ĵ�С��ϵ�� ��
�Ĵ�С��ϵ�� ����10�֣�
��1��0.125(2��)
��2��0.5mol-2��L-2 (2�֣���λ��д�۷�)
��3�������¶�(2��)������ѹǿ(2��)��K1<K2=K(2��)��
��1��0.125(2��)
��2��0.5mol-2��L-2 (2�֣���λ��д�۷�)
��3�������¶�(2��)������ѹǿ(2��)��K1<K2=K(2��)��
���������
��1���÷�Ӧ��0~8min��H2��ƽ����Ӧ������
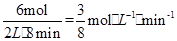 ����������֮�ȵ��ڼ�����֮��CO2��ƽ����Ӧ������0.125 mol��L-1��min-1
����������֮�ȵ��ڼ�����֮��CO2��ƽ����Ӧ������0.125 mol��L-1��min-1��2��ƽ�ⳣ��K=��
��3�����ߢ�Ϸ�ӦCO2��g����3H2��g��
 CH3OH��g����H2O��g��
CH3OH��g����H2O��g��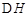 =-49��0kJ��mol���ı�����������¶�ʹ����ƽ������ʱ�����̵�������ת�����½������ߢ�ı����������ѹǿʹ����ƽ������ʱ�����̣�����ƽ�������ƶ�����ԭƽ�����n��H2�����٣�Kֻ���¶��йأ�
=-49��0kJ��mol���ı�����������¶�ʹ����ƽ������ʱ�����̵�������ת�����½������ߢ�ı����������ѹǿʹ����ƽ������ʱ�����̣�����ƽ�������ƶ�����ԭƽ�����n��H2�����٣�Kֻ���¶��йأ�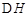 <0�ķ�Ӧ���¶�Խ�ߣ�KԽС������K1<K2=K��
<0�ķ�Ӧ���¶�Խ�ߣ�KԽС������K1<K2=K��
��ϰ��ϵ�д�
 ��ĩ�óɼ�ϵ�д�
��ĩ�óɼ�ϵ�д� 99��1������ĩ��ѵ��ϵ�д�
99��1������ĩ��ѵ��ϵ�д� ��ǿ��У��ĩ���100��ϵ�д�
��ǿ��У��ĩ���100��ϵ�д� �óɼ�1��1��ĩ���100��ϵ�д�
�óɼ�1��1��ĩ���100��ϵ�д� ��״Ԫ���źþ�ϵ�д�
��״Ԫ���źþ�ϵ�д�
�����Ŀ
 CH3OH(g)��H2O(g)����H����49.0 kJ��mol��1�����H2��CH3OH(g)��Ũ����ʱ��仯���±���ʾ������˵������ȷ����(����)
CH3OH(g)��H2O(g)����H����49.0 kJ��mol��1�����H2��CH3OH(g)��Ũ����ʱ��仯���±���ʾ������˵������ȷ����(����) 4NO+6H2O��2L���ܱ������н��У�����Ӻ�NO�����ʵ���������0.3mol����˷�Ӧ��ƽ������v (x)Ϊ�� ��
4NO+6H2O��2L���ܱ������н��У�����Ӻ�NO�����ʵ���������0.3mol����˷�Ӧ��ƽ������v (x)Ϊ�� �� 2Z(g)+2W(g)��2L�ܱ������н��У�5min��Y������0.5mol����˷�Ӧ������vΪ( )
2Z(g)+2W(g)��2L�ܱ������н��У�5min��Y������0.5mol����˷�Ӧ������vΪ( ) SO3(g)+NO(g) �ﵽƽ�⣬����Ӧ������ʱ��仯��ͼ��ʾ������ȷ�Ľ�����
SO3(g)+NO(g) �ﵽƽ�⣬����Ӧ������ʱ��仯��ͼ��ʾ������ȷ�Ľ�����
 2C��g��,���� 2 s���룩����C ��Ũ��Ϊ 0.6 mol��L��1 ���� ������ A ��ʾ�ķ�Ӧ��ƽ������ �� 2 s ʱ���� B ��Ũ��Ϊ ��
2C��g��,���� 2 s���룩����C ��Ũ��Ϊ 0.6 mol��L��1 ���� ������ A ��ʾ�ķ�Ӧ��ƽ������ �� 2 s ʱ���� B ��Ũ��Ϊ ��