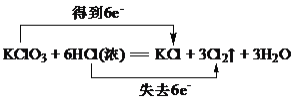ЬтФПФкШн
ЁОЬтФПЁПЕЈЗЏЃЈCuSO4ЁЄ5H2OЃЉгаЙуЗКЕФгУЭОЁЃФГбаОПадбЇЯАаЁзщРћгУФГДЮЪЕбщКѓЕФЯЁСђЫсЁЂЯЁЯѕЫсЛьКЯвКжЦБИЕЈЗЏЁЃЪЕбщСїГЬШчЯТЃК

ЧыЛиД№ЯТСаЮЪЬтЃК
ЃЈ1ЃЉВйзїXЮЊ___ЃЌ___ЁЃ
ЃЈ2ЃЉNOашвЊЛиЪеРћгУЃЌаДГіNOгыПеЦјЁЂH2OЗДгІЩњГЩЯѕЫсЕФЛЏбЇЗНГЬЪН___ЁЃ
ЃЈ3ЃЉЯжга48gКЌCuOжЪСПЗжЪ§ЮЊ20%ЕФЭЗлЃЌгывЛЖЈСПЕФЯЁСђЫсЁЂЯЁЯѕЫсЛьКЯвКЧЁКУЭъШЋЗДгІЩњГЩCuSO4ЁЃЪдЧѓЃК
ЂйРэТлЩЯЩњГЩЕЈЗЏЕФжЪСПЮЊ___gЁЃ
ЂкдЛьКЯвКжаСђЫсКЭЯѕЫсЕФЮяжЪЕФСПжЎБШЁЃ___ЃЈаДГіМЦЫуЙ§ГЬЃЉ
ЁОД№АИЁПеєЗЂХЈЫѕ РфШДНсОЇ 4NO+3O2+2H2O=4HNO3 180g ЭгыЯѕЫсЗДгІЕФРызгЗНГЬЪНЪЧ3Cu+8H++2NO3-=3Cu2++2NOЁќ+4H2O
дђЯћКФЕФЯѕЫсnЃЈHNO3ЃЉ=![]() 0.4molЃЌ
0.4molЃЌ
ИљОнСђдЊЫиЪиКуnЃЈH2SO4ЃЉ=nЃЈCuSO4ЁЄ5H2OЃЉ=0.72molЃЌ
дђnЃЈH2SO4ЃЉЃКnЃЈHNO3ЃЉ=0.72molЃК0.4mol=9ЃК5
ЁОНтЮіЁП
ЃЈ1ЃЉЭЗлгыЯЁСђЫсЁЂЯЁЯѕЫсЗДгІЩњГЩСђЫсЭШмвКЃЌНЋШмвКНјааеєЗЂХЈЫѕЁЂРфШДНсОЇВйзїЃЌЕУЕНОЇЬхЃЛ
ЃЈ2ЃЉNOгыПеЦјЁЂH2OЗДгІЩњГЩЯѕЫсЃЌНсКЯЕУЪЇЕчзгЪиКуЁЂжЪСПЪиКуХфЦНЗНГЬЪНЃЛ
ЃЈ3ЃЉМЦЫуCuOЁЂCuЕФжЪСПЃЌНсКЯЭдЊЫижЪСПЪиКуПЩМЦЫуCuSO4ЁЄ5H2OЕФжЪСПЃЛНсКЯЗДгІЕФРызгЗНГЬЪНМЦЫудЛьКЯвКжаСђЫсКЭЯѕЫсЕФЮяжЪЕФСПжЎБШЁЃ
ЃЈ1ЃЉЭЗлгыЯЁСђЫсЁЂЯЁЯѕЫсЗДгІЩњГЩСђЫсЭШмвКЃЌШчЕУЕНЕЈЗЏЃЌгІНЋШмвКНјааеєЗЂХЈЫѕЁЂРфШДНсОЇВйзїЃЌШЛКѓОЙ§ТЫЁЂЯДЕгЁЂИЩдяПЩЕУЕНОЇЬхЃЛ
ЃЈ2ЃЉNOгыПеЦјЁЂH2OЗДгІЩњГЩЯѕЫсЃЌЗДгІЕФЗНГЬЪНЮЊ4NO+3O2+2H2O=4HNO3ЃЛ
ЃЈ3ЃЉЂйnЃЈCuOЃЉ=![]() =0.12molЃЌ nЃЈCuЃЉ=
=0.12molЃЌ nЃЈCuЃЉ=![]() =0.6molЃЌ
=0.6molЃЌ
nЃЈCuSO4ЁЄ5H2OЃЉ=0.12mol+0.6mol=0.72molЃЛ
mЃЈCuSO4ЁЄ5H2OЃЉ=0.72molЁС250g/mol=180gЃЌРэТлЩЯЩњГЩЕЈЗЏЕФжЪСПЮЊ180gЃЛ
ЂкЭгыЯѕЫсЗДгІЕФРызгЗНГЬЪНЪЧ3Cu+8H++2NO3-=3Cu2++2NOЁќ+4H2O
дђЯћКФЕФЯѕЫсnЃЈHNO3ЃЉ=![]() 0.4molЃЌ
0.4molЃЌ
ИљОнСђдЊЫиЪиКуnЃЈH2SO4ЃЉ=nЃЈCuSO4ЁЄ5H2OЃЉ=0.72molЃЌ
дђnЃЈH2SO4ЃЉЃКnЃЈHNO3ЃЉ=0.72molЃК0.4mol=9ЃК5ЁЃ

 бєЙтПЮЬУПЮЪБзївЕЯЕСаД№АИ
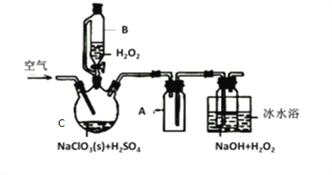
бєЙтПЮЬУПЮЪБзївЕЯЕСаД№АИЁОЬтФПЁПЫФТШЛЏЮ§ПЩгУзїУНШОМСЁЃРћгУШчЭМЫљЪОзАжУПЩвджЦБИЫФТШЛЏЮ§ЃЈВПЗжМаГжзАжУвбТдШЅЃЉ

гаЙиаХЯЂШчЯТБэЃК
ЛЏбЇЪН | SnCl2 | SnCl4 |
ШлЕу/Ёц | 246 | 33 |
ЗаЕу/Ёц | 652 | 144 |
ЦфЫћаджЪ | ЮоЩЋОЇЬхЃЌвзбѕЛЏ | ЮоЩЋвКЬхЃЌвзЫЎНт |
ЛиД№ЯТСаЮЪЬтЃК
ЃЈ1ЃЉМззАжУжавЧЦїAЕФУћГЦЮЊ___________ЁЃ
ЃЈ2ЃЉгУМззАжУжЦТШЦјЃЌMnO4БЛЛЙдЮЊMn2+ЃЌИУЗДгІЕФРызгЗНГЬЪНЮЊ________________ЁЃ
ЃЈ3ЃЉНЋзАжУШчЭМСЌНгКУЃЌМьВщЦјУмадЃЌТ§Т§ЕЮШыХЈбЮЫсЃЌД§ЙлВьЕН__________ЃЈЬюЯжЯѓЃЉКѓЃЌПЊЪММгШШЖЁзАжУЃЌЮ§ШлЛЏКѓЪЪЕБдіДѓТШЦјСїСПЃЌМЬајМгШШЖЁзАжУЃЌДЫЪБМЬајМгШШЖЁзАжУЕФФПЕФЪЧЃКЂйДйНјТШЦјгыЮ§ЗДгІЃЛЂк_______________________ЁЃ
ЃЈ4ЃЉввзАжУЕФзїгУ____________ЃЌШчЙћШБЩйввзАжУЃЌПЩФмЗЂЩњЕФИБЗДгІЕФЛЏбЇЗНГЬЪНЮЊ_________ЃЛМКзАжУЕФзїгУЪЧ_____ЃЈЬюађКХЃЉЁЃ
AЃЎЗРжЙПеЦјжаCO2ЦјЬхНјШыЮьзАжУ
BЃЎГ§ШЅЮДЗДгІЕФТШЦјЃЌЗРжЙЮлШОПеЦј
CЃЎЗРжЙЫЎеєЦјНјШыЮьзАжУЕФЪдЙмжаЪЙВњЮяЫЎНт
DЃЎЗРжЙПеЦјжаO2НјШыЮьзАжУЕФЪдЙмжаЪЙВњЮябѕЛЏ
ЃЈ5ЃЉФГЭЌбЇШЯЮЊЖЁзАжУжаЕФЗДгІПЩФмВњЩњSnCl2дгжЪЃЌвдЯТЪдМСжаВЛПЩгУгкМьВтЪЧЗёВњЩњSnCl2 ЕФга_______ЃЈЬюађКХЃЉЁЃ
AЃЎH2O2ШмвК BЃЎЫсадИпУЬЫсМиШмвК CЃЎAgNO3ШмвК DЃЎфхЫЎ
ЃЈ6ЃЉЗДгІжагУШЅЮ§СЃ1.19 gЃЌЗДгІКѓдкЮьзАжУЕФЪдЙмжаЪеМЏЕН2.04 g SnCl4ЃЌдђSnCl4ЕФВњТЪЮЊ_______ЃЈБЃСє2ЮЛгааЇЪ§зжЃЉЁЃ