��Ŀ����
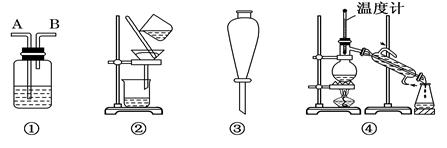
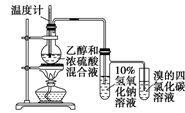
��֪ij��ȼ�Ϻ���̼���⡢������Ԫ�ء�Ϊ�˲ⶨ����ȼ����̼��������Ԫ�ص������ȣ��ɽ���̬ȼ�Ϸ���������������ȼ�գ���ʹ����������ȫ��ͨ����ͼ��ʾ��װ�ã��õ����±����е�ʵ����(���������������ȫ������)��
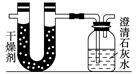
����ʵ��������
(1)ʵ����Ϻ���������ˮ������Ϊ________ g��
������ƿ������һ�����Σ�������Ϊ________ g��
(2)���ɵ�ˮ����Ԫ�ص�����Ϊ________ g��
(3)���ɵĶ�����̼��̼Ԫ�ص�����Ϊ________ g��
(4)��ȼ����̼Ԫ������Ԫ�ص�������Ϊ________��
(5)��֪���ִ���ÿ�������к���һ����ԭ�ӣ���ô��ķ���ʽΪ__________���ṹ��ʽΪ_______________________________��

| | ʵ��ǰ | ʵ��� |
| (�������U�ι�)������ | 101.1 g | 102.9 g |
| (ʯ��ˮ�����ƿ)������ | 312.0 g | 314.2 g |
����ʵ��������
(1)ʵ����Ϻ���������ˮ������Ϊ________ g��
������ƿ������һ�����Σ�������Ϊ________ g��
(2)���ɵ�ˮ����Ԫ�ص�����Ϊ________ g��
(3)���ɵĶ�����̼��̼Ԫ�ص�����Ϊ________ g��
(4)��ȼ����̼Ԫ������Ԫ�ص�������Ϊ________��
(5)��֪���ִ���ÿ�������к���һ����ԭ�ӣ���ô��ķ���ʽΪ__________���ṹ��ʽΪ_______________________________��
(1)1.8��5��(2)0.2��(3)0.6��(4)3��1
(5)CH4O��CH3OH
(5)CH4O��CH3OH
(1)m(H2O)��102.9 g��101.1 g��1.8 g
m(CO2)��314.2 g��312.0 g��2.2 g
��n(CaCO3)��n(CO2)��0.05 mol
m(CaCO3)��5 g
(2)m (H)��m(H2O)��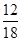 ��1.8 g��
��1.8 g��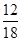 ��0.2 g
��0.2 g
(3)m(C)��m(CO2)��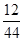 ��2.2 g��
��2.2 g��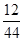 ��0.6 g
��0.6 g
(4)m(C)��m(H)��0.6 g��0.2 g��3��1
(5)��ȼ�Ϸ�����C��H��ԭ�Ӹ�����Ϊ��
N(C)��N(H)��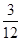 ��
�� ��1��4��
��1��4��
��̼�ļ۵�ԭ���֪�����л�������е�̼��ԭ�Ӹ�����Ϊ1��4ʱ��������ֻ�ܺ�CH4��������ΪCH4��������������Ϊÿ�������к���һ����ԭ�ӣ���ô��ķ���ʽΪCH4O���ṹ��ʽΪCH3OH��
m(CO2)��314.2 g��312.0 g��2.2 g
��n(CaCO3)��n(CO2)��0.05 mol
m(CaCO3)��5 g
(2)m (H)��m(H2O)��
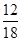 ��1.8 g��
��1.8 g��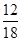 ��0.2 g
��0.2 g(3)m(C)��m(CO2)��
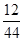 ��2.2 g��
��2.2 g��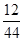 ��0.6 g
��0.6 g(4)m(C)��m(H)��0.6 g��0.2 g��3��1
(5)��ȼ�Ϸ�����C��H��ԭ�Ӹ�����Ϊ��
N(C)��N(H)��
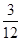 ��
�� ��1��4��
��1��4����̼�ļ۵�ԭ���֪�����л�������е�̼��ԭ�Ӹ�����Ϊ1��4ʱ��������ֻ�ܺ�CH4��������ΪCH4��������������Ϊÿ�������к���һ����ԭ�ӣ���ô��ķ���ʽΪCH4O���ṹ��ʽΪCH3OH��

��ϰ��ϵ�д�
�����Ŀ