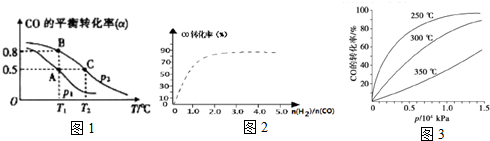
��Ŀ����
7������ֲ���纣���������к��зḻ�ĵ�Ԫ�أ���Ԫ���Ե����ӵ���ʽ���ڣ�ʵ������Ӻ�������ȡ���������ͼ��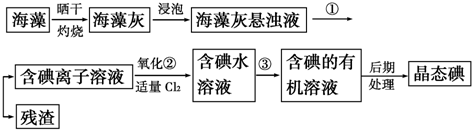
��1��ָ����ȡ��Ĺ������йص�ʵ��������ƣ��ٹ��ˣ�����ȡ����Һ��
��2����ȡ��Ĺ����У��ɹ�ѡ����л��Լ���BD��
A���ƾ� B�����Ȼ�̼
C������ D��ֱ������
��3��Ϊʹ������е�����ת��Ϊ����л���Һ��ʵ���������ձ���������������ƿ���ƾ��ơ����ܡ�Բ����ƿ��ʯ�����Լ���Ҫ�ļг���������Ʒ����ȱ�ٵIJ��������ǣ���ͨ��©���ͷ�Һ©����
���� ����ɹ�����տ�����ˮ�Ͷ�����̼���������к��е⻯�ص����ʣ�����ˮ�����ݹ��˵õ����е����ӵ���Һ��������ˮ��ͨ�������������û����⣬�õ����ˮ��Һ�������Ȼ�̼��ȡ�õ�������л���Һ��������ɵõ��⣬�Դ˽����⣮
��1����������Һ���ù��ˣ���һ���ܼ������ʴ�������һ���ܼ�����ɵ���Һ����ȡ��������ȡ����ȡ�Ļ���ԭ�������ܼ��������ܣ���������һ���ܼ��е��ܽ�ȱ�����һ�ִ�Ķࣻ
��2����ȡ�Ļ���ԭ�������ܼ��������ܣ���������һ���ܼ��е��ܽ�ȱ�����һ�ִ�Ķ࣬�ݴ���ѡ����ʵ��Լ���
��3����������װ��ͼ�����жϣ�����ʵ��ԭ��ѡ����ʵ�������
��� �⣺��1���ٷ�������Һ���ù��ˣ����������ڻ������ܵ��ܼ����ܽ�Ȳ�ͬ����һ���ܼ������ʴ�������һ���ܼ�����ɵ���Һ����ȡ���������ַ���������ȡ���۽���ˮ�еĵⵥ����ȡ�������������ܵ����ʵķ����Ƿ�Һ����
�ʴ�Ϊ�����ˣ���ȡ��Һ��
��2����ȡ�Ļ���ԭ�������ܼ��������ܣ���������һ���ܼ��е��ܽ�ȱ�����һ�ִ�Ķ࣬�ʿ������Ȼ�̼��ֱ�����ͣ��ƾ�������Ⱥ�ˮ�ǻ��ܵģ�����ѡ��
�ʴ�Ϊ��BD��
��3������ʵ��װ��ԭ����ʹ�õ������У��ձ���������������ƿ���ƾ��ơ����ܡ�Բ����ƿ��ʯ������©������Һ©���ȣ��ʴ�Ϊ������ͨ��©���ͷ�Һ©����
���� ���⿼�����ʵķ�����ᴿ��ѧϰ��ע�����չ��ˡ���ȡ������Ȳ���Ҫ�㣬��Ŀ�ѶȲ���

| A�� | C2H5OH+3O2=2CO2+3H2O��H=-29.7 kJ/mol | |
| B�� | C2H5OH��l��+3O2 ��g��=2CO2 ��g��+3H2O��l����H=+1366.2 kJ/mol | |
| C�� | C2H5OH��l��+3O2 ��g��=2CO2 ��g��+3H2O��g����H=+29.7 kJ/mol | |
| D�� | C2H5OH��l��+3O2 ��g��=2CO2 ��g��+3H2O��l����H=-1366.2 kJ/mol |
�ٻ��������ܶ� �ڻ�������ѹǿ ��B�����ʵ���Ũ�� ������������ʵ��� �ݻ�������ƽ����Է���������
| A�� | �٢ڢ� | B�� | �٢ܢ� | C�� | �٢ۢ� | D�� | �ڢۢ� |
| A�� | ���� | B�� | ԭ������ | C�� | �������� | D�� | �ܶ� |
| A�� | ��84������Һ | B�� | ��ˮ | C�� | Cl2 | D�� | Ư�۾���Һ |
| A�� | ��Na2CO3��Һ�У�c��OH-��-c��H+��=c��HCO3-��+c��H2CO3�� | |
| B�� | 0.2mol•L?1��CH3COONa��Һ��0.1mol•L?1��HCl��Һ�������ϣ���Һ�����ԣ�c��Na+����c��Cl-����c��CH3COO-����c��H+����c��OH-�� | |
| C�� | pH��ͬ��̼������Һ��̼��������Һ��c��NaHCO3����c��Na2CO3�� | |
| D�� | 0.1mol•L?1��HCN�����ᣩ��0.1mol•L?1��NaCN�������ϣ�c��HCN��+c��CN-��=c��Na+�� |