��Ŀ����
ʵ������ȡ���������ܶ࣬ͨ�����������֣�
��1���ö���������Ũ���Ṳ����ȡ�������������������������20 mL 12 mol/L��Ũ�����ϼ��ȣ���ַ�Ӧ�����ɵ�������������0.06 mol�������ý������Ҫԭ���ǣ��� ���� ��
��2�����������Ũ���ᷴӦ����ȡ�������䷴Ӧ�Ļ�ѧ����ʽΪ��
��
������0.1 mol���������������Ļ�ԭ�������ʵ���Ϊ mol��
��3���ְ����²��������й��������Ʊ�������ʵ�飺��һƬ�³İ�ֽ��Բ�β���Ƭ�ϣ�����ͼ����A���һ��0.1 mol/L KI��Һ����������Һ����B���һ��FeSO4����KSCN����Һ��C���һ��NaOH������̪����Һ��O�������KClO3���塣��KClO3����μ�һ��Ũ���ᣬ�����ñ�����Ǻá��Իش��������⣺
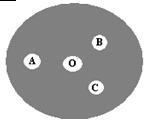
��A�㷴Ӧ�����ӷ���ʽΪ ��
��B�������Ϊ ��
��C�����Һ�ɺ�ɫ��Ϊ��ɫ������Ϊ�к���ɫ����Ư����ɫ���������ʵ��֤��֮�� ��
��1���ö���������Ũ���Ṳ����ȡ�������������������������20 mL 12 mol/L��Ũ�����ϼ��ȣ���ַ�Ӧ�����ɵ�������������0.06 mol�������ý������Ҫԭ���ǣ��� ���� ��
��2�����������Ũ���ᷴӦ����ȡ�������䷴Ӧ�Ļ�ѧ����ʽΪ��
��
������0.1 mol���������������Ļ�ԭ�������ʵ���Ϊ mol��
��3���ְ����²��������й��������Ʊ�������ʵ�飺��һƬ�³İ�ֽ��Բ�β���Ƭ�ϣ�����ͼ����A���һ��0.1 mol/L KI��Һ����������Һ����B���һ��FeSO4����KSCN����Һ��C���һ��NaOH������̪����Һ��O�������KClO3���塣��KClO3����μ�һ��Ũ���ᣬ�����ñ�����Ǻá��Իش��������⣺

��A�㷴Ӧ�����ӷ���ʽΪ ��
��B�������Ϊ ��
��C�����Һ�ɺ�ɫ��Ϊ��ɫ������Ϊ�к���ɫ����Ư����ɫ���������ʵ��֤��֮�� ��

��

��ϰ��ϵ�д�
 ����Ӣ��ϵ�д�
����Ӣ��ϵ�д� ����ѧУ�ֲ����ܲ�ϵ�д�
����ѧУ�ֲ����ܲ�ϵ�д� �ƸԺ���ȫ�����Ų��Ծ�ϵ�д�
�ƸԺ���ȫ�����Ų��Ծ�ϵ�д�
�����Ŀ

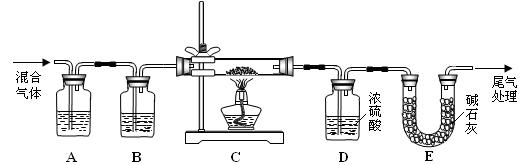
 CO�� + CO2�� + H2O������װ���У�����������ֽ���ȡ������� �� ��������ĸ��
CO�� + CO2�� + H2O������װ���У�����������ֽ���ȡ������� �� ��������ĸ��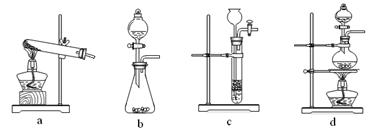
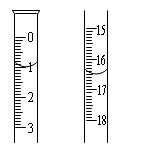
 ���еIJ����ǣ���a�����װ�������ԣ���b�� �� ��
���еIJ����ǣ���a�����װ�������ԣ���b�� �� ��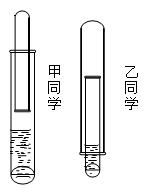
 MnCl2+Cl2��+2H2O
MnCl2+Cl2��+2H2O