��Ŀ����
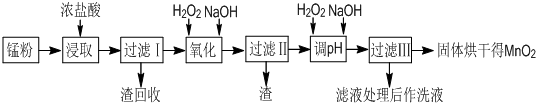
��16�֣����յķϾ�п�̸ɵ�ؾ���������ɵõ��̷ۣ���Ҫ��MnO2��Mn(OH)2��Fe��NH4Cl��̿�ڵȣ������̷���ȡMnO2�IJ�����������£�
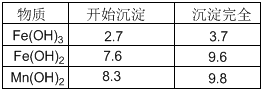
| ���� | ��ʼ���� | ������ȫ |
| Fe(OH)3 | 2.7 | 3.7 |
| Fe(OH)2 | 7.6 | 9.6 |
| Fe(OH)2 | 8.3 | 9.8 |
��2������I�������Ļ�ѧʽ ������I�������Ļ�ѧʽ ��
��3������I����Һ�м�����H2O2������Ϊ ���ټ�NaOH��Һ����pH��3.7<pH<8.3��Ŀ���� ��
��4������II����Һ��H2O2��Һ���ټ�NaOH��Һ����pHΪ9��ʹMn2+ת����MnO2������һ������H2O2�� ��������������ԭ���������á�
��1��MnO2+4H++2Cl-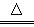
��2��C��2�֣���Fe(OH)2��2�� ��
��
��3����Fe2+ת��ΪFe3+��2�֣�ʹFe3+��ȫ������1�֣�����ֹMn2+ת��ΪMn(OH)2������2�֣�
��4����������2�֣�
����

��16�֣����յķϾ�п�̸ɵ�ؾ���������ɵõ��̷ۣ���Ҫ��MnO2��Mn(OH)2��Fe��NH4Cl��̿�ڵȣ������̷���ȡMnO2�IJ�����������£�
| ���� | ��ʼ���� | ������ȫ |
| Fe(OH)3 | 2.7 | 3.7 |
| Fe(OH)2 | 7.6 | 9.6 |
| Fe(OH)2 | 8.3 | 9.8 |
��1���ڼ��������£���Ũ�����ȡ�̷۵õ�����Mn2+��Fe3+�����ӵ���Һ��MnO2��Ũ���ᷴӦ�����ӷ���ʽ ���ô���������ȱ�� ��
��2������I�������Ļ�ѧʽ ������I�������Ļ�ѧʽ ��
��3������I����Һ�м�����H2O2������Ϊ ���ټ�NaOH��Һ����pH��3.7<pH<8.3��Ŀ���� ��
��4������II����Һ��H2O2��Һ���ټ�NaOH��Һ����pHΪ9��ʹMn2+ת����MnO2������һ������H2O2�� ��������������ԭ���������á�
���յķϾ�п�̸ɵ�ؾ���������õ��̷�(��MnO2��Mn(OH)2��Fe����Ȳ�ͺ�̿��)�����̷���ȡMnO2�IJ�������ͼ��ʾ��
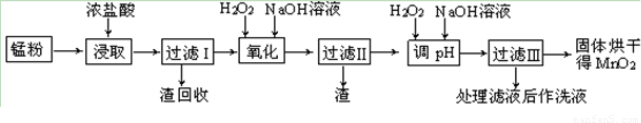
������ͼ��ʾ���貢�ο��������ݣ��ش��������⡣
�� �� | ��ʼ���� | ������ȫ |
Fe(OH)3 | 2.7 | 3.7 |
Fe(OH)2 | 7.6 | 9.6 |
Mn(OH)2 | 8.3 | 9.8 |
��1����������������Ũ�����ȡ�̷ۣ�������Һ�к���Mn2+��Fe2+�ȡ�MnO2��Ũ���ᷴӦ�����ӷ��̷���ʽ��?????????????????????????????????????????????????????? _��
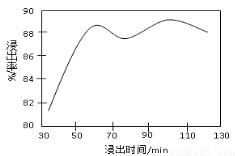
��2�����ʱ������ʱ����̽����ʵ�Ӱ������ͼ��ʾ����ҵ���õ��ǽ�ȡ60 min�������ԭ����???????????????????????????????????????????????????? ��
��3���̷۾�Ũ�����ȡ������I��ȥ�������ʺ�����Һ�м�������H2O2��Һ����������????????????????????????????????? ��
��4������I������Һ�������������NaOH��Һ����pHԼΪ5.1����Ŀ����?????????????????????????????????????????????? ��
��5��������������Һ��������H2O2��Һ������NaOH��Һ����pHԼΪ9��ʹMn2+ �����õ�MnO2����Ӧ�����ڷ���ʽΪ????????????????????????????????????????????????????? ��
��6����ҵ������KOH��MnO2Ϊԭ����ȡKMnO4����Ҫ�������̷��������У���һ����MnO2����KOH���飬��Ͼ��ȣ��ڿ����м������ۻ�����������������ȡK2MnO4���ڶ���Ϊ���K2MnO4��Ũ��Һ��ȡKMnO4��
�� ��һ����Ӧ�Ļ�ѧ����ʽΪ???????????????????????????????????????????????????????????? ��
�� ���K2MnO4��Ũ��Һʱ��������������ʵ������Ϊ???????????????????????????????????????? ��
��16�֣����յķϾ�п�̸ɵ�ؾ���������ɵõ��̷ۣ���Ҫ��MnO2��Mn(OH)2��Fe��NH4Cl��̿�ڵȣ������̷���ȡMnO2�IJ�����������£�

|
���� |
��ʼ���� |
������ȫ |
|
Fe(OH)3 |
2.7 |
3.7 |
|
Fe(OH)2 |
7.6 |
9.6 |
|
Fe(OH)2 |
8.3 |
9.8 |
��1���ڼ��������£���Ũ�����ȡ�̷۵õ�����Mn2+��Fe3+�����ӵ���Һ��MnO2��Ũ���ᷴӦ�����ӷ���ʽ ���ô���������ȱ�� ��
��2������I�������Ļ�ѧʽ ������I�������Ļ�ѧʽ ��
��3������I����Һ�м�����H2O2������Ϊ ���ټ�NaOH��Һ����pH��3.7<pH<8.3��Ŀ���� ��
��4������II����Һ��H2O2��Һ���ټ�NaOH��Һ����pHΪ9��ʹMn2+ת����MnO2������һ������H2O2�� ��������������ԭ���������á�