��Ŀ����
����Ŀ����ͼ����4��Բ�����ɵ�Ԫ�ظ���Ĺ�ϵͼ������4Ϊ���Բ��3��2��1���μ�С�����жϷ������ֹ�ϵ����

A. 1�������Ԫ�ء�2������Ԫ�ء�3����ҪԪ�ء�4������Ԫ��
B. 1������Ԫ�ء�2����ҪԪ�ء�3������Ԫ�ء�4�������Ԫ��
C. 1������Ԫ�ء�2�������Ԫ�ء�3������Ԫ�ء�4����ҪԪ��
D. 1����ҪԪ�ء�2������Ԫ�ء�3�������Ԫ�ء�4������Ԫ��
���𰸡�A
��������
�����ĺ�ѡ���������֪�����⿼��ѧ����ϸ���е�Ԫ�ص����֪ʶ��ʶ�Ǻ�����������
C�������Ԫ�أ�C��H��O��N�ǻ���Ԫ�أ�C��H��O��N��P��S����ҪԪ�أ�����Ԫ�ذ���C��H��O��N��P��S��K��Ca��Mg�ȣ��ݴ��������ͼʾ������֪��A��ȷ��B��C��D������

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ����T��ʱ,������ӦC(s)+H2O(g) ![]() CO(g)+H2(g)��
CO(g)+H2(g)��
��1��T��ʱ,��һ��2L���ܱ������м���4molC��1molH2O(g),5min��Ӧ�ﵽƽ��,C��ת����Ϊ20%��
��0��5min��,��H2O(g)��ʾ��ƽ����Ӧ����Ϊ____________��
�ڸ÷�Ӧ�ﵽƽ��ı�־��______��
a.�����ƽ����Է�����������
b.������ܶȲ���
c.H2O(g)��CO(g)��H2(g)�����ʵ���֮��Ϊ1��1:1
d.����������ʵ�������
��2��T��ʱ,�������ܱ������м�����Ӧ�����ʽ���ʵ��,�м�ĸ���������ɻ�����

���������������ƽ����Է�������һֱ���ֲ��䣬��Ӧ��ʼǰH2O(g)��H2(g)�����ʵ���֮����__________��
�ڷ�Ӧ�ڴﵽƽ��ʱ,�м�ĸ���������λ����________��
a.1��1.5֮�� b.1.5�� c.1.5��2֮��
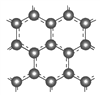
��3����֪:I.�ƻ�1mol���ۼ�����Ҫ���������±���
���ۼ� | ʯī�е�̼̼�� | H-H | C��O | H-O |
����/kJ | 475.7 | 436 | 1072 | 463 |
��.ʯī���������η䳲�ṹ��̼ԭ�ӹ��ɣ���ͼ��ʾ:
ijͬѧ����:ͨ������װ��ʵ��C(s)+H2O(g) ![]() CO(g)+H2(g)�ķ�Ӧ��
CO(g)+H2(g)�ķ�Ӧ��
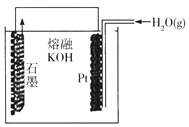
�ٰ��ÿ����Ĺ���,ʯī�缫�����ķ�ӦʽΪ_______________��
��������������?˵������: _______________��
����Ŀ�����ص����������������ǵij��м���������˼����Σ�����о�NO2��SO2��CO�ȴ�����Ⱦ������γɼ�����������Ҫ���塣
(1)500��ʱ���ڴ������������£��ֱ�2molSO2��1molO2���ں�ѹ�����ͺ�����������(��������ʼ�ݻ���ͬ)����ַ�Ӧ�����߾��ﵽƽ���:
����������SO2��ת���ʹ�ϵ�Ǽ�_____��(�>������<����=��)��
�����������У���Ӧ�ﵽƽ��ı�������������ʹSO2��ת������ߵ���____(����ĸ)��
a.�¶Ⱥ�����������䣬����1.0molHe b.�¶Ⱥ�����������䣬����1.0molO2
c.��������������ʱ������1molSO3 d.��������������ʱ�����ø�Ч����
(2)�����Ƽ�ѭ�������ѳ������е�SO2��
�����Ƽ�ѭ�����У�Na2SO3��Һ����Ϊ����Һ������NaOH��Һ����SO2�Ƶã��÷�Ӧ�����ӷ���ʽ��_______________��
������Һ����SO2�Ĺ����У�pH��n(SO32-):n(HSO3-)�仯��ϵ���±�:
n(SO32-):n(HSO3-) | 91:9 | 1:1 | 9:91 |
pH | 8.2 | 7.2 | 6.2 |
���ϱ��жϣ�NaHSO3��Һ��_____��(��ᡱ��������С�)���û�ѧƽ��ԭ������:________��
(3)��CH4������ԭNO2������������������Ⱦ������:
CH4(g)+4NO2(g)=4NO(g)+CO2(g)+2H2O(g) ��H=-574kJ/mol
CH4(g)+4NO(g)=2N2(g)+CO2(g)+2H2O(g) ��H=-1160kJ/mol
���ñ�״����4.48CH4��ԭNO2��N2������������ת�Ƶĵ�������Ϊ_____(�����ӵ�������ֵ��NA��ʾ)���ų�������Ϊ_______kJ��
(4)��ҵ�Ϻϳɰ������������Ʊ������У����е�һ����ӦΪ��CO(g)+H2O(g)====CO2(g)+H2(g) ��H<0��һ�������£���CO(g)��H2O(g)�������Ϊ1��2�����ܱ������з���������Ӧ���ﵽƽ��ʱ���CO(g)��H2O(g)�����Ϊ1:6.��ƽ�ⳣ��K=______(������������λС��)��