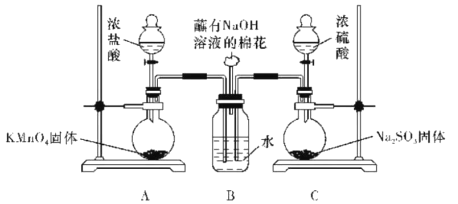
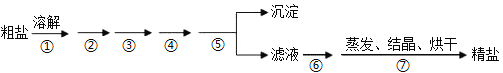
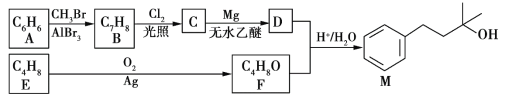
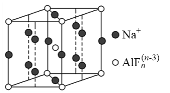
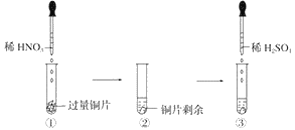
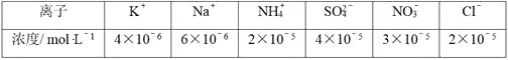
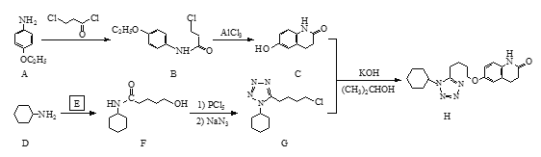
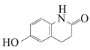
��Ŀ����
����Ŀ����֪H������д��H3O+����ijЩ���ʿ���NH3��H2O��![]() ��H3O����OH����
��H3O����OH����![]() ��N3����O2���������ƣ��ݴ��ж����з�Ӧʽ(��Ӧ��������)��ȷ����(����)
��N3����O2���������ƣ��ݴ��ж����з�Ӧʽ(��Ӧ��������)��ȷ����(����)
��2Na��2NH3=2NaNH2��H2��
��CaO��2NH4Cl=CaCl2��2NH3����H2O
��3Mg(NH2)2=Mg3N2��4NH3��
��NH4Cl��NaNH2=NaCl��2NH3��
A.��B.�ڢ�C.ȫ��D.�٢ڢ�
���𰸡�C
��������
��ijЩ���ʿ���NH3��H2O��NH4+��H3O+��OH-��NH2-��N3-��O2-�������ƣ�������Ϣ�������Ʒ��������жϡ�
��NH3��H2O���ƣ���������2Na+2H2O�T2NaOH+H2�������Ƶõ���ӦΪ��2Na+2NH3�T2NaNH2+H2�����ʢ���ȷ��
��NH4+��H3O+���ƣ�H������д��H3O+������CaO+2HCl=CaCl2+H2O�����Ƶõ���Ӧ��CaO+2NH4Cl=CaCl2+2NH3��+H2O���ʢ���ȷ��
��OH-��NH2-���ƣ�N3-��O2-���ƣ�NH3��H2O���ƣ�����Mg(OH)2=MgO+H2O�����Ƶõ���3Mg(NH2)2=Mg3N2+4NH3�����ʢ���ȷ��
��OH-��NH2-���ƣ�NH3��H2O���ƣ�����NH4Cl+NaOH=NaCl+NH3+H2O�����Ƶõ���NH4Cl+NaNH2�TNaCl+2NH3���ʢ���ȷ��
��ѡC��

 �ܿ�����ĩ��̾�ϵ�д�
�ܿ�����ĩ��̾�ϵ�д�