��Ŀ����
ͭ��ұ�����̴��¿ɷ�Ϊ���ٸ��������������и�ѡ���ڱ��գ���Ҫ��ӦΪ
2CuFeS2+4O2 ="=" Cu2S+3SO2+2FeO��¯���������ƴ�ͭ����1200�淢������Ҫ��ӦΪ�� 2Cu2S+3O2 ="=" 2Cu2O+2SO2��2Cu2O+Cu2S ="=" 6Cu+SO2�����ܵ�⾫��ͭ������˵������ȷ���ǣ� ��
2CuFeS2+4O2 ="=" Cu2S+3SO2+2FeO��¯���������ƴ�ͭ����1200�淢������Ҫ��ӦΪ�� 2Cu2S+3O2 ="=" 2Cu2O+2SO2��2Cu2O+Cu2S ="=" 6Cu+SO2�����ܵ�⾫��ͭ������˵������ȷ���ǣ� ��
| A��Cu2O�Ǻ�ɫ���� |
| B��ұ�������е�β�������������� |
| C�����������У���2 mol CuFeS2��ȡCuʱ����������5.0mol O2 |
| D����⾫��ͭ�Ŀ�ʼ�Σ�ÿת��2mol����ʱ�������ܽ�ͭ������Ϊ64g |
D
��

��ϰ��ϵ�д�
 ���ſ����ϵ�д�
���ſ����ϵ�д� ���Ŀ����ϵ�д�
���Ŀ����ϵ�д� ������ӱ������ͯ������ϵ�д�
������ӱ������ͯ������ϵ�д�
�����Ŀ
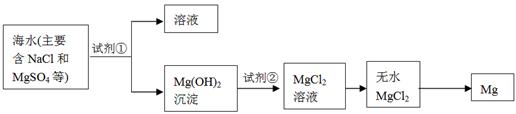

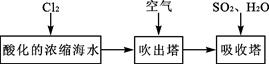
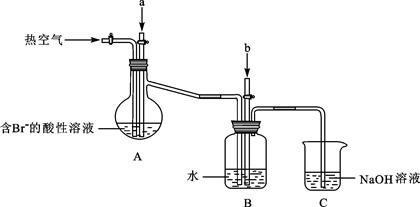
 CH3OH(g) ��H1
CH3OH(g) ��H1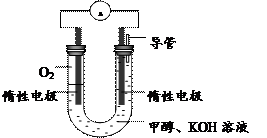 ��3��ijʵ��С�����ݼ״�ȼ�յķ�Ӧԭ����
��3��ijʵ��С�����ݼ״�ȼ�յķ�Ӧԭ���� ���ٶ���ʻ��40km��Ϊ����Ч��չ��������Դ�����ȱ����Ƶ����۵����������мȿ����־��õ����ⷽ���� �������������� ����д��ţ�
���ٶ���ʻ��40km��Ϊ����Ч��չ��������Դ�����ȱ����Ƶ����۵����������мȿ����־��õ����ⷽ���� �������������� ����д��ţ� ��4��������������ȼ������ˮ���Ȼ�ѧ����ʽ
��4��������������ȼ������ˮ���Ȼ�ѧ����ʽ