��Ŀ����
1���о���Ԫ�ؼ��仯��������ʾ�����Ҫ���壮��������ͼװ��ģ�ҵ������SO2�������ķ�Ӧ���о�SO2�����ʣ�
���۵㣺SO2-76.1�棬SO3 16.8�棻�е㣺SO2-10�棬SO3 45�棩
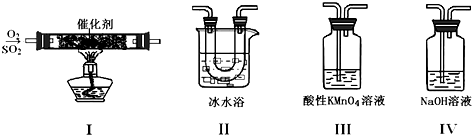
��1����ͬѧ��I��II��III��IV��˳������װ�ã�װ��II��������ʹSO3����ɹ�����SO2���룻װ��III����Һ����ɫ��֤������������л�ԭ�ԣ�
��2����ͬѧ������˳������װ�ã�װ�â��г����ȴ������װ�â�����40mL 3.0mol/L NaOH��Һ����Ӧ������5.12g����װ�â��з�����Ӧ�Ļ�ѧ����ʽ��2SO2+3NaOH=Na2SO3+NaHSO3+H2O��
��3��ijͬѧ��������SO2ͨ��һ֧װ���Ȼ�����Һ���Թ��У�δ���������ɣ�����Թ��м�������ABD������ĸ�������ܲ���������
A��������Һ B����ˮ C������ D���������Һ
��Ϊ�о����ʲ�������Ũ����ķ�Ӧ��ijѧϰС�����������̽�����
��ȡ������̼�ظ֣�6g����15mLŨ�����У����ȣ���ַ�Ӧ��õ���ҺX���ռ�������Y��
��4����ͬѧ��ΪX�г�Fe3+����ܺ���Fe2+����Ҫȷ�����е�Fe2+Ӧѡ��C��ѡ����ţ���
a��KSCN��Һ����ˮ b������������Һ
c������KMnO4��Һ d�����ۺ�KSCN��Һ
��5����ͬѧȡ784mL����״��������Yͨ������H2O2ˮ��Һ�У�Ȼ���������BaCl2��Һ�����ʵ�������ø������4.66g���ɴ���֪����Y��SO2������ٷ���Ϊ57.1%��������С�����һλ��
��6����������ʵ����SO2��������Ľ������ͬѧ��Ϊ����Y�л����ܺ���H2��C02���壬����C02��������C+2H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$CO2��+2SO2��+2H2O���û�ѧ����ʽ��ʾ����
���� ��1��װ��II����������ȴװ�ã���������ķе�ϵͣ�ͨ����ˮ����ʹSO3����ɹ�����SO2���룬װ��III�и��������Һ��Ͷ�������Ӧ����ɫ������Mn2+��������������Ϊ���
��2����װ��IV����40mL 3mol•L-1NaOH��Һ����Ӧ������5.12g�����յ�Ϊ�����������壬���ݶ����������ʵ����������������ʵ������з����ж����ɲ����n��SO2����n��NaOH��=1��2��Ӧ���շ�Ӧ��SO2+2NaOH=Na2SO3+H2O����n��SO2����n��NaOH��=1��1����Ӧ���շ�ӦSO2+NaOH=NaHSO3��װ��IV����40mL 3mol•L-1NaOH��Һ��n��NaOH��=0.12mol��n��SO2��=$\frac{5.12g}{64g/mol}$=0.08mol��n��SO2����n��NaOH��=0.08��0.12=2��3���������ɲ���Ϊ�������ƺ����������ƣ���Ӧ�Ļ�ѧ����ʽ����ֱƽ��д����2SO2+3NaOH�TNa2SO3+NaHSO3+H2O��
��3��SO2�ڼ��������»�������������������Ȼ����ܲ���������
��4������+2������������ʹ�ữ�ĸ��������ɫ�����飻
��5�����ɵ�SO2���л�ԭ�ԣ�ͨ���������������з�����Ӧ��SO2+2H2O2=2H2O+H2SO4��������Ԫ�ص��غ㲢��Ϲ�ϵʽ��SO2��BaSO4���SO2�����ʵ������ټ����������������������
��6����������ʵ����SO2��������Ľ������ͬѧ��Ϊ����Y�л����ܺ���H2��C02���壬����C02��������̼��Ũ������ȷ�Ӧ���ɶ�����̼�Ͷ�������
��� �⣺��1��װ��II����������ȴװ�ã���������ķе�ϵͣ�ͨ����ˮ����ʹSO3����ɹ�����SO2���룬װ��III�и��������Һ��Ͷ�������Ӧ����ɫ������Mn2+��������������Ϊ���ᣬ�����˶�������Ļ�ԭ�ԣ���Ӧ�����ӷ���ʽΪ��5SO2+2H2O+2MnO4-=5SO42-+2Mn2++4H+��
�ʴ�Ϊ��ʹSO3����ɹ�����SO2���룬��ԭ�ԣ�
��2����װ��IV����40mL 3mol•L-1NaOH��Һ����Ӧ������5.12g�����յ�Ϊ�����������壬���ݶ����������ʵ����������������ʵ������з����ж����ɲ����n��SO2����n��NaOH��=1��2��Ӧ���շ�Ӧ��SO2+2NaOH=Na2SO3+H2O����n��SO2����n��NaOH��=1��1����Ӧ���շ�ӦSO2+NaOH=NaHSO3��װ��IV����40mL 3mol•L-1NaOH��Һ��n��NaOH��=0.12mol��n��SO2��=$\frac{5.12g}{64g/mol}$=0.08mol��n��SO2����n��NaOH��=0.08��0.12=2��3���������ɲ���Ϊ�������ƺ����������ƣ���Ӧ�Ļ�ѧ����ʽ����ֱƽ��д����2SO2+3NaOH�TNa2SO3+NaHSO3+H2O��
�ʴ�Ϊ��2SO2+3NaOH=Na2SO3+NaHSO3+H2O��
��3��A������������Һ��������������Ʒ�Ӧ�������ʳ�������A��ȷ��
B�����Թ�����백ˮ���������백ˮ��Ӧ����������泥�����������Ȼ��������������ᱵ��ɫ��������B��ȷ��
C��������SO2ͨ��װ���Ȼ�����Һ���Թܣ��ټ�ϡ����������������C����
D��������SO2ͨ��װ���Ȼ�����Һ���Թܣ���Һ�����ԣ�������е���������������������£���ǿ�����ԣ����Խ�SO2������������������Ȼ����������ᱵ��ɫ��������D��ȷ��
�ʴ�Ϊ��ABD��
��4��+2������������ʹ�ữ�ĸ��������ɫ��a��d�����ܼ����������ӣ�ֻ��C�ܼ�����Һ�к���+2�������ӣ�ѡb���������ֳ�����������������ɫ��Ӱ�������ֻ��ѡC��
�ʴ�Ϊ��C��
��5��SO2���л�ԭ�ԣ�ͨ���������������з�����Ӧ��SO2+2H2O2=2H2O+H2SO4��
�����784mL�����������ʵ���Ϊ��n��������壩=$\frac{0.784L}{22.4mol/L}$=0.035mol��
SO2��BaSO4
1mol 233g
n 4.66g
��n=$\frac{1mol��4.66g}{233g/mol}$=0.02mol��
�ʻ��������SO2���������Ϊ��$\frac{0.02mol}{0.035mol}$��100%��57.1%��
�ʴ�Ϊ��57.1%��
��6����������ʵ����SO2��������Ľ������ͬѧ��Ϊ����Y�л����ܺ���H2��C02���壬����C02�������Dz���C02��������̼��Ũ������ȷ�Ӧ���ɶ�����̼�Ͷ�������Ӧ�Ļ�ѧ����ʽΪ��C+2H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$CO2��+2SO2��+2H2O��
�ʴ�Ϊ��C+2H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$CO2��+2SO2��+2H2O��
���� �����ۺϿ���Ԫ�ػ�����֪ʶ��������貢���ʵ�鷽�������������ֱ�����������Ŀ�Ѷ��еȣ�������Խ�ǿ���漰Ũ�����ǿ�����ԣ�C��S��Fe���仯��������ʣ�ע����������Ũ��������ʼ�����ʵ�鷽�������������ԭ��

 �ο�������ϵ�д�
�ο�������ϵ�д� ������ѧ��ʱ��ҵϵ�д�
������ѧ��ʱ��ҵϵ�д� ���������ʱ��ѵϵ�д�
���������ʱ��ѵϵ�д� �㽭�¿γ���άĿ�������ʱ��ѵϵ�д�
�㽭�¿γ���άĿ�������ʱ��ѵϵ�д�| ʵ��Ŀ�� | �Լ������� |
| ֤���ع����е��� | B |
| ��ȥCO2�л��е�����CO | D |
| ����FeCl2��Һ�Ƿ���� | C |
| ��ȥNa2CO3������NaHCO3 | A |
A������
B���μӵ�ˮ
C������KSCN��Һ
D��ͨ�����ȵ�CuO��
��6.72L CH4����3.01��1023��HCl���Ӣ�13.6g H2S����0.2mol NH3��
| A�� | ������ܣ��٣��ڣ��� | B�� | �ܶȣ��ڣ��ۣ��ܣ��� | ||
| C�� | �������ڣ��ۣ��٣��� | D�� | ��ԭ�������٣��ڣ��ۣ��� |
| A�� | �������Ƶĵ���ʽ��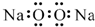 | B�� | ������Ľṹʽ��H-Cl-O | ||
| C�� | ��ԭ�ӵĽṹʾ��ͼ��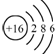 | D�� | NH4Cl�ĵ���ʽ�� |
| A�� | ��������������ˮ���� | |
| B�� | 40%�ļ�ȩˮ��Һ����������������������걾 | |
| C�� | ��ҵ�ƾ��������������þ� | |
| D�� | ú��������Һ����Ҫ���������ȼ�ϣ�ú�ĸ�����Ҫ����������ԭ�� |
| A�� | ������Ȼ�̼�γɵ���Һ����������ķ��������� | |
| B�� | ����ȡ�ķ����������ͺ�ú�� | |
| C�� | ���ܽ���˵ķ�����������غ��Ȼ��ƹ������� | |
| D�� | ��O2��H2�Ļ������ͨ�����ȵ�����ͭ���Գ�ȥ���е�H2 |
| A�� | ��ͬѧ���ü��ȵķ����ɳ�ȥKNO3��Һ�л��е�Fe3+��˵����Fe3+����ˮ�������ȵ� | |
| B�� | ��ͬѧ��������茶�������ˮ����ˮ���½���˵�������ˮ�������ȵ� | |
| C�� | ��ͬѧ��ͨ��ʵ�鷢��ͬŨ�ȵ��ȵĴ�����Һ����Ĵ�����Һȥ����Ч���ã�˵��̼�� ��ˮ�������ȵ� | |
| D�� | ��ͬѧ���ڴ�������Һ�е����̪��Һ�����ȣ�������ˮ������������ɫ���˵��������ˮ�������ȵ� |
 �� ��2-��-1-��ϩ�ļ���ʽ
�� ��2-��-1-��ϩ�ļ���ʽ ��
��